CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
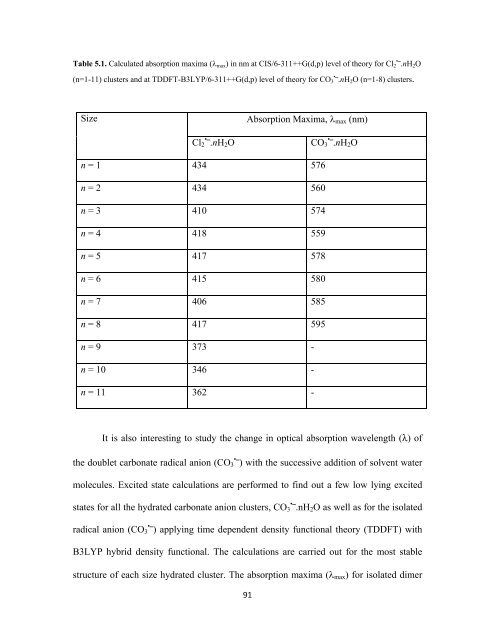
Table 5.1. Calculated absorption maxima (λ max ) in nm at CIS/6-311++G(d,p) level of theory for Cl • 2 ⎯.nH 2 O<br />
(n=1-11) clusters and at TDDFT-B3LYP/6-311++G(d,p) level of theory for CO • 3 ⎯.nH 2 O (n=1-8) clusters.<br />
Size<br />
Absorption Maxima, λ max (nm)<br />
Cl 2 • ⎯.nH 2 O<br />
CO 3 • ⎯.nH 2 O<br />
n = 1 434 576<br />
n = 2 434 560<br />
n = 3 410 574<br />
n = 4 418 559<br />
n = 5 417 578<br />
n = 6 415 580<br />
n = 7 406 585<br />
n = 8 417 595<br />
n = 9 373 -<br />
n = 10 346 -<br />
n = 11 362 -<br />
It is also interesting to study the change in optical absorption wavelength (l) of<br />
the doublet carbonate radical anion (CO • 3 ⎯) with the successive addition of solvent water<br />
molecules. Excited state calculations are performed to find out a few low lying excited<br />
states for all the hydrated carbonate anion clusters, CO • 3 ⎯.nH 2 O as well as for the isolated<br />
radical anion (CO • 3 ⎯) applying time dependent density functional theory (TDDFT) with<br />
B3LYP hybrid density functional. The calculations are carried out for the most stable<br />
structure of each size hydrated cluster. The absorption maxima (λ max ) for isolated dimer<br />
91