CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
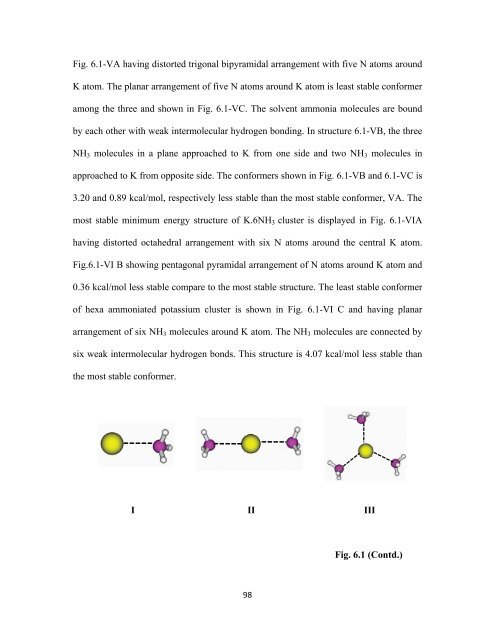
Fig. 6.1-VA having distorted trigonal bipyramidal arrangement with five N atoms around<br />
K atom. The planar arrangement of five N atoms around K atom is least stable conformer<br />
among the three and shown in Fig. 6.1-VC. The solvent ammonia molecules are bound<br />
by each other with weak intermolecular hydrogen bonding. In structure 6.1-VB, the three<br />
NH 3 molecules in a plane approached to K from one side and two NH 3 molecules in<br />
approached to K from opposite side. The conformers shown in Fig. 6.1-VB and 6.1-VC is<br />
3.20 and 0.89 kcal/mol, respectively less stable than the most stable conformer, VA. The<br />
most stable minimum energy structure of K.6NH 3 cluster is displayed in Fig. 6.1-VIA<br />
having distorted octahedral arrangement with six N atoms around the central K atom.<br />
Fig.6.1-VI B showing pentagonal pyramidal arrangement of N atoms around K atom and<br />
0.36 kcal/mol less stable compare to the most stable structure. The least stable conformer<br />
of hexa ammoniated potassium cluster is shown in Fig. 6.1-VI C and having planar<br />
arrangement of six NH 3 molecules around K atom. The NH 3 molecules are connected by<br />
six weak intermolecular hydrogen bonds. This structure is 4.07 kcal/mol less stable than<br />
the most stable conformer.<br />
I II III<br />
Fig. 6.1 (Contd.)<br />
98