CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Here, the symbols are used to denote intensity (I), frequency (ω), Plank constant (h )<br />
inverse of Boltzmann constant multiplied by temperature (β ) 1/kT), time (t), and dipole<br />
moment (μ).<br />
3.3. Results and Discussion<br />
The clusters, X. nH 2 O (X= Cl 2 •− , Br 2 •− , I 2 •− , CO 3 •− , NO 3<br />
−<br />
and CO 3 2− ) are<br />
stabilized by the SHB, DHB and WHB interactions as discussed in Chapter 2. Due to<br />
these interactions it is expected that bands due to O-H stretching and bending modes of<br />
H 2 O in the hydrated clusters of X get shifted compared to that of free water molecule.<br />
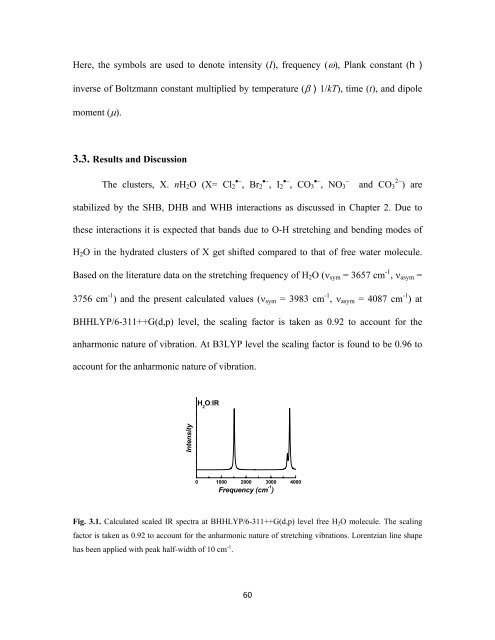
Based on the literature data on the stretching frequency of H 2 O (ν sym = 3657 cm -1 , ν asym =<br />
3756 cm -1 ) and the present calculated values (ν sym = 3983 cm -1 , ν asym = 4087 cm -1 ) at<br />
BHHLYP/6-311++G(d,p) level, the scaling factor is taken as 0.92 to account for the<br />
anharmonic nature of vibration. At B3LYP level the scaling factor is found to be 0.96 to<br />
account for the anharmonic nature of vibration.<br />
H 2<br />
O:IR<br />
Intensity<br />
0 1000 2000 3000 4000<br />
Frequency (cm -1 )<br />
Fig. 3.1. Calculated scaled IR spectra at BHHLYP/6-311++G(d,p) level free H 2 O molecule. The scaling<br />
factor is taken as 0.92 to account for the anharmonic nature of stretching vibrations. Lorentzian line shape<br />
has been applied with peak half-width of 10 cm -1 .<br />
60