CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
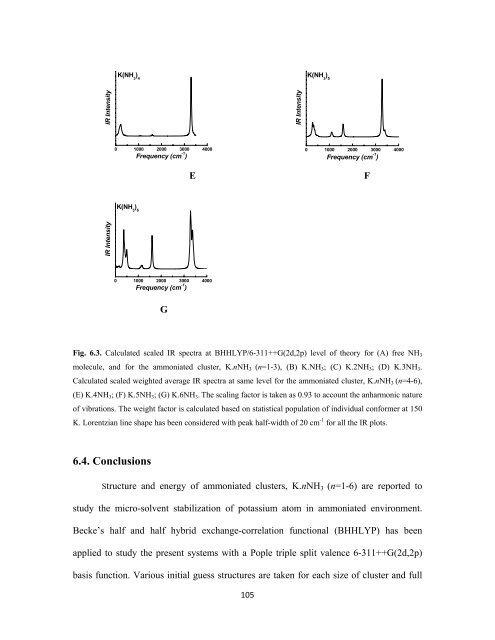
K(NH 3<br />
) 4<br />
K(NH 3<br />
) 5<br />
IR Intensity<br />
IR Intensity<br />
0 1000 2000 3000 4000<br />
Frequency (cm -1 )<br />
E<br />
0 1000 2000 3000 4000<br />
Frequency (cm -1 )<br />
F<br />
K(NH 3<br />
) 6<br />
IR Intensity<br />
0 1000 2000 3000 4000<br />
Frequency (cm -1 )<br />
G<br />
Fig. 6.3. Calculated scaled IR spectra at BHHLYP/6-311++G(2d,2p) level of theory for (A) free NH 3<br />
molecule, and for the ammoniated cluster, K.nNH 3 (n=1-3), (B) K.NH 3 ; (C) K.2NH 3 ; (D) K.3NH 3 .<br />
Calculated scaled weighted average IR spectra at same level for the ammoniated cluster, K.nNH 3 (n=4-6),<br />
(E) K.4NH 3 ; (F) K.5NH 3 ; (G) K.6NH 3 . The scaling factor is taken as 0.93 to account the anharmonic nature<br />
of vibrations. The weight factor is calculated based on statistical population of individual conformer at 150<br />
K. Lorentzian line shape has been considered with peak half-width of 20 cm -1 for all the IR plots.<br />
6.4. Conclusions<br />
Structure and energy of ammoniated clusters, K.nNH 3 (n=1-6) are reported to<br />
study the micro-solvent stabilization of potassium atom in ammoniated environment.<br />
Becke’s half and half hybrid exchange-correlation functional (BHHLYP) has been<br />
applied to study the present systems with a Pople triple split valence 6-311++G(2d,2p)<br />
basis function. Various initial guess structures are taken for each size of cluster and full<br />
105