CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
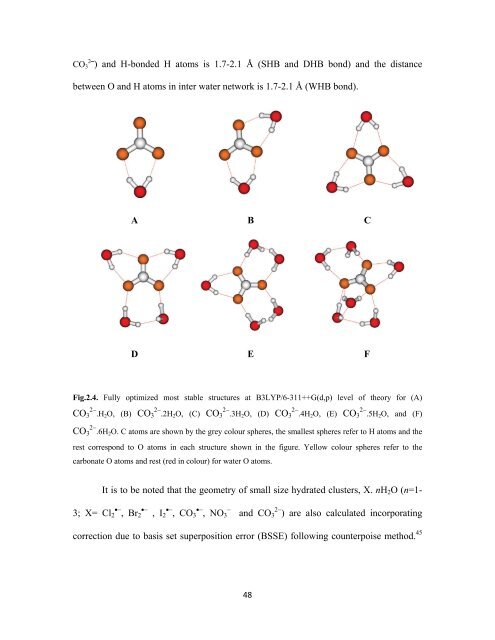
CO 3 2 ⎯) and H-bonded H atoms is 1.7-2.1 Å (SHB and DHB bond) and the distance<br />
between O and H atoms in inter water network is 1.7-2.1 Å (WHB bond).<br />
A B C<br />
D E F<br />
Fig.2.4. Fully optimized most stable structures at B3LYP/6-311++G(d,p) level of theory for (A)<br />
2− 2− 2− 2− 2−<br />
CO 3 .H2 O, (B) CO 3 .2H2 O, (C) CO 3 .3H2 O, (D) CO 3 .4H2 O, (E) CO 3 .5H2 O, and (F)<br />
2−<br />
CO 3 .6H2 O. C atoms are shown by the grey colour spheres, the smallest spheres refer to H atoms and the<br />
rest correspond to O atoms in each structure shown in the figure. Yellow colour spheres refer to the<br />
carbonate O atoms and rest (red in colour) for water O atoms.<br />
It is to be noted that the geometry of small size hydrated clusters, X. nH 2 O (n=1-<br />
3; X= Cl 2 •− , Br 2 •− , I 2 •− , CO 3 •− , NO 3<br />
−<br />
and CO 3 2− ) are also calculated incorporating<br />
correction due to basis set superposition error (BSSE) following counterpoise method. 45<br />
48