Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
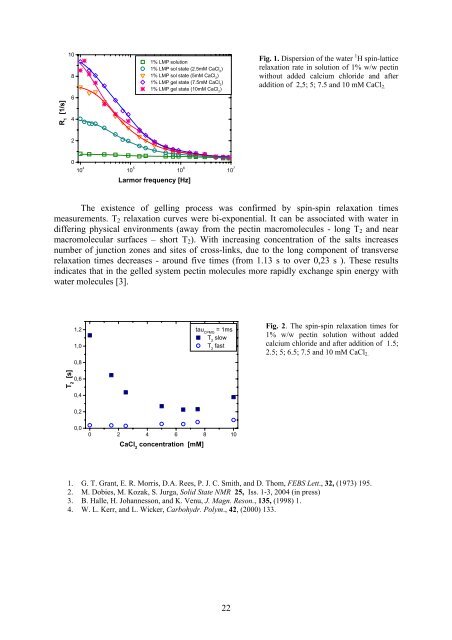
R 1<br />
[1/s]<br />
10<br />
8<br />
6<br />
4<br />
1% LMP solution<br />
1% LMP sol state (2.5mM CaCl 2<br />
)<br />
1% LMP sol state (5mM CaCl 2<br />
)<br />
1% LMP gel state (7.5mM CaCl 2<br />
)<br />
1% LMP gel state (10mM CaCl 2<br />
)<br />
Fig. 1. Dispersion of the water 1 H spin-lattice<br />
relaxation rate in solution of 1% w/w pectin<br />
without added calcium chloride and after<br />
addition of 2,5; 5; 7.5 and 10 mM CaCl 2.<br />
2<br />
0<br />
10 4 10 5 10 6 10 7<br />
Larmor frequency [Hz]<br />
The existence of gelling process was confirmed by spin-spin relaxation times<br />
measurements. T 2 relaxation curves were bi-exponential. It can be associated with water in<br />
differing physical environments (away from the pectin macromolecules - long T 2 and near<br />
macromolecular surfaces – short T 2 ). With increasing concentration of the salts increases<br />
number of junction zones and sites of cross-links, due to the long component of transverse<br />
relaxation times decreases - around five times (from 1.13 s to over 0,23 s ). These results<br />
indicates that in the gelled system pectin molecules more rapidly exchange spin energy with<br />
water molecules [3].<br />
1,2 tau CPMG<br />
= 1ms<br />
T 2<br />
slow<br />
1,0<br />
T 2<br />
fast<br />
0,8<br />
Fig. 2. The spin-spin relaxation times for<br />
1% w/w pectin solution without added<br />
calcium chloride and after addition of 1.5;<br />
2.5; 5; 6.5; 7.5 and 10 mM CaCl 2.<br />
T 2<br />
[s]<br />
0,6<br />
0,4<br />
0,2<br />
0,0<br />
0 2 4 6 8 10<br />
CaCl 2<br />
concentration [mM]<br />
1. G. T. Grant, E. R. Morris, D.A. Rees, P. J. C. Smith, and D. Thom, FEBS Lett., 32, (1973) 195.<br />
2. M. Dobies, M. Kozak, S. Jurga, Solid State NMR 25, Iss. 1-3, 2004 (in press)<br />
3. B. Halle, H. Johannesson, and K. Venu, J. Magn. Reson., 135, (1998) 1.<br />
4. W. L. Kerr, and L. Wicker, Carbohydr. Polym., 42, (2000) 133.<br />
22