Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
DSC measurements confirm existing of L-alanine / Polyethylene Glycol complex.<br />
Melting temperature for the complex (578K) is lower then that for pure L-Alanine (588K),<br />
and in the DSC diagram, due to low concentration, is not observed a melting peak for the pure<br />
Polyethylene Glycol.<br />
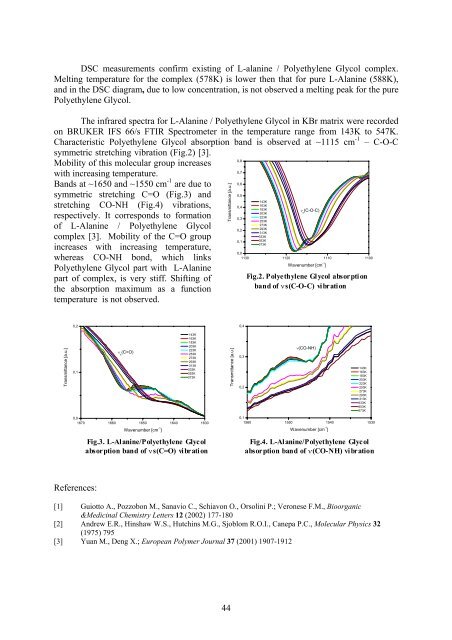
The infrared spectra for L-Alanine / Polyethylene Glycol in KBr matrix were recorded<br />
on BRUKER IFS 66/s FTIR Spectrometer in the temperature range from 143K to 547K.<br />
Characteristic Polyethylene Glycol absorption band is observed at ~1115 cm -1 – C-O-C<br />
symmetric stretching vibration (Fig.2) [3].<br />
0,8<br />
Mobility of this molecular group increases<br />
0,7<br />
with increasing temperature.<br />
Bands at ~1650 and ~1550 cm -1 are due to<br />
0,6<br />
symmetric stretching C=O (Fig.3) and<br />
0,5<br />
143K<br />
stretching CO-NH (Fig.4) vibrations,<br />
163K<br />
0,4<br />
183K<br />
ν<br />
203K<br />
s<br />
(C-O-C)<br />
respectively. It corresponds to formation<br />
0,3<br />
223K<br />
253K<br />
273K<br />
of L-Alanine / Polyethylene Glycol<br />
0,2<br />
293K<br />
313K<br />
complex [3]. Mobility of the C=O group<br />
333K<br />
0,1<br />
353K<br />
373K<br />
increases with increasing temperature,<br />
0,0<br />
whereas CO-NH bond, which links<br />
1130 1120 1110 1100<br />
Wavenumber [cm<br />
Polyethylene Glycol part with L-Alanine<br />
-1 ]<br />
part of complex, is very stiff. Shifting of Fig.2. Polyethylene Glycol absorption<br />
the absorption maximum as a function<br />
band of νs(C-O-C) vibration<br />
temperature is not observed.<br />
Transmittance [a.u.]<br />
Transmittance [a.u.]<br />
0,2<br />
0,1<br />
ν s<br />
(C=O)<br />
0,0<br />
1670 1660 1650 1640 1630<br />
Wavenumber [cm -1 ]<br />
143K<br />
163K<br />
183K<br />
203K<br />
223K<br />
253K<br />
273K<br />
293K<br />
313K<br />
333K<br />
353K<br />
373K<br />
Fig.3. L-Alanine/Polyethylene Glycol<br />
absorption band of νs(C=O) vibration<br />
Transmittance [a.u.]<br />
0,4<br />
0,3<br />
0,2<br />
ν(CO-NH)<br />
143K<br />
163K<br />
183K<br />
203K<br />
223K<br />
253K<br />
273K<br />
293K<br />
313K<br />
333K<br />
353K<br />
373K<br />
0,1<br />
1560 1550 1540 1530<br />
Wavenumber [cm -1 ]<br />
Fig.4. L-Alanine/Polyethylene Glycol<br />
absorption band of ν(CO-NH) vibration<br />
References:<br />
[1] Guiotto A., Pozzobon M., Sanavio C., Schiavon O., Orsolini P.; Veronese F.M., Bioorganic<br />
&Medicinal Chemistry Letters 12 (2002) 177-180<br />
[2] Andrew E.R., Hinshaw W.S., Hutchins M.G., Sjoblom R.O.I., Canepa P.C., Molecular Physics 32<br />
(1975) 795<br />
[3] Yuan M., Deng X.; European Polymer Journal 37 (2001) 1907-1912<br />
44