Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
STUDIES OF POLY(ASPARTIC ACID) STRUCTURE AND ITS<br />
DERIVATIVES USING 1 H NMR SPECTROSCOPY<br />
Elżbieta Tylek, Jolanta Polaczek, Jan Pielichowski<br />
Department of Chemistry and Technology oh Polymers Technical University, Kraków, Poland<br />
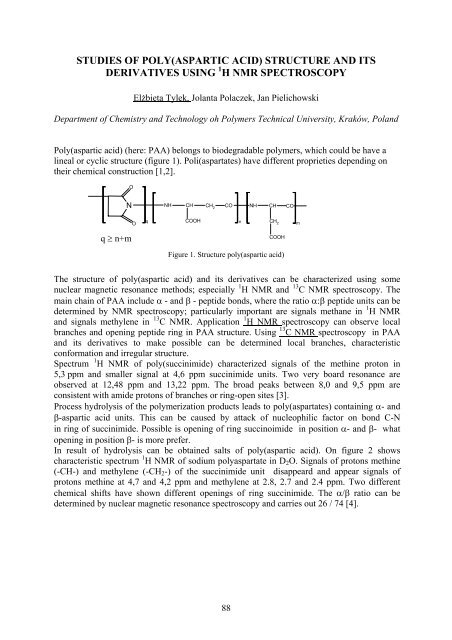
Poly(aspartic acid) ( here: PAA) belongs to biodegradable polymers, which could be have a<br />
lineal or cyclic structure (figure 1). Poli(aspartates) have different proprieties depending on<br />
th eir chemical construction [1,2].<br />
O<br />
N<br />
NH<br />
CH<br />
CH 2<br />
CO NH CH<br />
CO<br />
O<br />
q<br />
COOH<br />
n<br />
CH 2<br />
m<br />
q ≥ n+m<br />
COOH<br />
Figure 1. Structure poly(aspartic acid)<br />
The structure of poly(aspartic acid) and its derivatives can be characterized using some<br />
1<br />
nuclear magnetic resonance methods; especially H NMR and 13 C NMR spectroscopy. The<br />
main chain of PAA include α - and β - peptide bonds, where the ratio α:β peptide units can be<br />
determined by NMR spectroscopy; particularly important are signals methane in 1 H NMR<br />
and signals methylene in 13 C NMR. Application 1 H NMR spectroscopy can observe local<br />
branches and opening peptide ring in PAA structure. Using 13 C NMR spectroscopy in PAA<br />
and its derivatives to make possible can be determined local branches, characteristic<br />
conformation and irregular structure.<br />
Spectrum 1 H NMR of poly(succinimide) characterized signals of the methine proton in<br />
5,3 ppm and smaller signal at 4,6 ppm succinimide units. Two very board resonance are<br />
observed at 12,48 ppm and 13,22 ppm. The broad peaks between 8,0 and 9,5 ppm are<br />
consistent with amide protons of branches or ring-open sites [3].<br />
Process hydrolysis of the polymerization products leads to poly(aspartates) containing α- and<br />
β-as partic acid units. This can be caused by attack of nucleophilic factor on bond C-N<br />
in ring of succinimide. Possible is opening of ring succinoimide in position α- and β- what<br />
opening in position β- is more prefer.<br />
In result of hydrolysis can be obtained salts of poly(aspartic acid). On figure 2 shows<br />
characteristic spectrum 1 H NMR of sodium polyaspartate in D 2 O. Signals of protons methine<br />
(-CH-) and methylene (-CH 2 -) of the succinimide unit disappeard and appear signals of<br />
protons methine at 4,7 and 4,2 ppm and methylene at 2.8, 2.7 and 2.4 ppm. Two different<br />
chemical shifts have shown different openings of ring succinimide. The α/β ratio can be<br />
determined by nuclear magnetic resonance spectroscopy and carries out 26 / 74 [4].<br />
88