Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
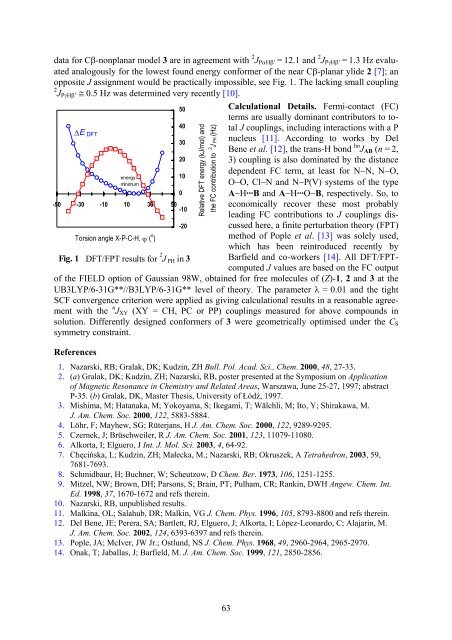
data for Cβ-nonplanar model 3 are in agreement with 2 J PαHβ’ = 12.1 and 2 J PγHβ’ = 1.3 Hz evaluated<br />
analogously for the lowest found energy conformer of the near Cβ-planar ylide 2 [7]; an<br />
opposite J assignment would be practically impossible, see Fig. 1. The lacking small coupling<br />
2 J PγHβ’ ≅ 0.5 Hz was determined very recently [10].<br />
∆E DFT<br />
FC term<br />
energy<br />
minimum<br />
-50 -30 -10 10 30 50<br />
-10<br />
Torsion angle X-P-C-H, ϕ ( o )<br />
-20<br />
Fig. 1 DFT/FPT results for 2 J PH in 3<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
Relative DFT energy (kJ/mol) and<br />
the FC contribution to 2 J PH (Hz)<br />
Calculational Details. Fermi-contact (FC)<br />
terms are usually dominant contributors to total<br />
J couplings, including interactions with a P<br />
nucleus [11]. According to works by Del<br />
Bene et al. [12], the trans-H bond hn J AB (n = 2,<br />
3) coupling is also dominated by the distance<br />
dependent FC term, at least for N−N, N−O,<br />
O−O, Cl−N and N−P(V) systems of the type<br />
A−H···B and A−H···O−B, respectively. So, to<br />
economically recover these most probably<br />
leading FC contributions to J couplings discussed<br />
here, a finite perturbation theory (FPT)<br />
method of Pople et al. [13] was solely used,<br />
which has been reintroduced recently by<br />
Barfield and co-workers [14]. All DFT/FPTcomputed<br />
J values are based on the FC output<br />
of the FIELD option of Gaussian 98W, obtained for free molecules of (Z)-1, 2 and 3 at the<br />
UB3LYP/6-31G**//B3LYP/6-31G** level of theory. The parameter λ = 0.01 and the tight<br />
SCF convergence criterion were applied as giving calculational results in a reasonable agreement<br />
with the n J XY (XY = CH, PC or PP) couplings measured for above compounds in<br />
solution. Differently designed conformers of 3 were geometrically optimised under the C S<br />
symmetry constraint.<br />
References<br />
1. Nazarski, RB; Gralak, DK; Kudzin, ZH Bull. Pol. Acad. Sci., Chem. 2000, 48, 27-33.<br />
2. (a) Gralak, DK; Kudzin, ZH; Nazarski, RB, poster presented at the Symposium on Application<br />
of Magnetic Resonance in Chemistry and Related Areas, Warszawa, June 25-27, 1997; abstract<br />
P-35. (b) Gralak, DK, Master Thesis, University of Łódź, 1997.<br />
3. Mishima, M; Hatanaka, M; Yokoyama, S; Ikegami, T; Wälchli, M; Ito, Y; Shirakawa, M.<br />
J. Am. Chem. Soc. 2000, 122, 5883-5884.<br />
4. Löhr, F; Mayhew, SG; Rüterjans, H J. Am. Chem. Soc. 2000, 122, 9289-9295.<br />
5. Czernek, J; Brüschweiler, R J. Am. Chem. Soc. 2001, 123, 11079-11080.<br />
6. Alkorta, I; Elguero, J Int. J. Mol. Sci. 2003, 4, 64-92.<br />
7. Chęcińska, L; Kudzin, ZH; Małecka, M.; Nazarski, RB; Okruszek, A Tetrahedron, 2003, 59,<br />
7681-7693.<br />
8. Schmidbaur, H; Buchner, W; Scheutzow, D Chem. Ber. 1973, 106, 1251-1255.<br />
9. Mitzel, NW; Brown, DH; Parsons, S; Brain, PT; Pulham, CR; Rankin, DWH Angew. Chem. Int.<br />
Ed. 1998, 37, 1670-1672 and refs therein.<br />
10. Nazarski, RB, unpublished results.<br />
11. Malkina, OL; Salahub, DR; Malkin, VG J. Chem. Phys. 1996, 105, 8793-8800 and refs therein.<br />
12. Del Bene, JE; Perera, SA; Bartlett, RJ, Elguero, J; Alkorta, I; López-Leonardo, C; Alajarin, M.<br />
J. Am. Chem. Soc. 2002, 124, 6393-6397 and refs therein.<br />
13. Pople, JA; McIver, JW Jr.; Ostlund, NS J. Chem. Phys. 1968, 49, 2960-2964, 2965-2970.<br />
14. Onak, T; Jaballas, J; Barfield, M. J. Am. Chem. Soc. 1999, 121, 2850-2856.<br />
63