Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1 H, 13 C NMR AND GIAO/DFT CALCULATIONS OF SUBSTITUTED<br />
N-(4-ARYL-1-PIPERAZINYLBUTYL) DERIVATIVES,<br />
NEW ANALOGUES OF BUSPIRONE<br />
Maciej Pisklak a , Jerzy Kossakowski b , Iwona Wawer a<br />
a Department of Physical Chemistry, Faculty of Pharmacy, The Medical University<br />
of Warsaw, Banacha 1, 02097 Warsaw, Poland; b Department of Medicinal Chemistry,<br />
The Medical University of Warsaw, Oczki 3, 02007 Warsaw, Poland<br />
Buspirone was a first of the anxiolytics of second generation, with high affinity to<br />
5-HT1A and D2 receptors. The ligands for the 5-HT1A receptors are of special interest since<br />
they may be important in treating psychiatric disorders (anxiety, depressions). Several groups<br />
of ligands have been synthesised and tested for pharmacological activity with the task to find<br />
a compound with better selectivity and higher affinity. Continuing our studies on potential<br />
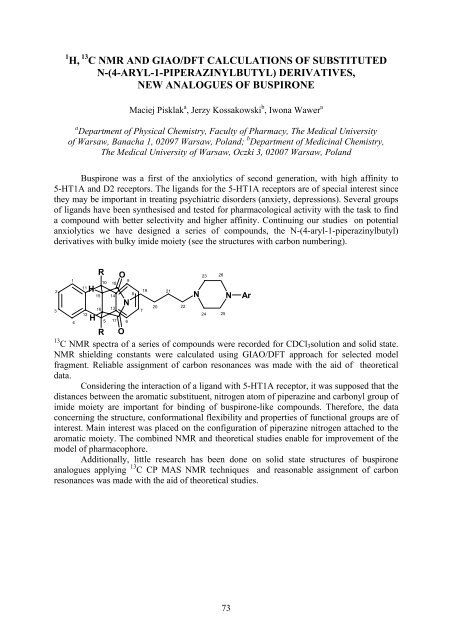
anxiolytics we have designed a series of compounds, the N-(4-aryl-1-piperazinylbutyl)<br />
derivatives with bulky imide moiety (see the structures with carbon numbering).<br />
2<br />
3<br />
1<br />
11<br />
H<br />
15<br />
R<br />
10<br />
18<br />
14<br />
O<br />
N<br />
16 13<br />
12<br />
H<br />
4 5 17 6<br />
9<br />
8<br />
7<br />
19<br />
20<br />
21<br />
22<br />
N<br />
23<br />
26<br />
24 25<br />
N<br />
R O<br />
13 C NMR spectra of a series of compounds were recorded for CDCl 3 solution and solid state.<br />
NMR shielding constants were calculated using GIAO/DFT approach for selected model<br />
fragment. Reliable assignment of carbon resonances was made with the aid of theoretical<br />
data.<br />
Considering the interaction of a ligand with 5-HT1A receptor, it was supposed that the<br />
distances between the aromatic substituent, nitrogen atom of piperazine and carbonyl group of<br />
imide moiety are important for binding of buspirone-like compounds. Therefore, the data<br />
concerning the structure, conformational flexibility and properties of functional groups are of<br />
interest. Main interest was placed on the configuration of piperazine nitrogen attached to the<br />
aromatic moiety. The combined NMR and theoretical studies enable for improvement of the<br />
model of pharmacophore.<br />
Additionally, little research has been done on solid state structures of buspirone<br />
analogues applying 13 C CP MAS NMR techniques and reasonable assignment of carbon<br />
resonances was made with the aid of theoretical studies.<br />
Ar<br />
73