Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ESTIMATION OF h2 J PH AND 2 J PH SCALAR COUPLINGS<br />
BY DFT/FPT METHOD TO RATIONALISE TWO DISTINCT<br />
NMR OBSERVATIONS<br />
Ryszard B. Nazarski<br />
Department of Organic Chemistry, Institute of Chemistry, University of Łódź,<br />
90-950 Łódź 1, P.O. Box 376, Poland. E-mail: rynaz@chemul.uni.lodz.pl<br />
Four years ago, our report appeared [1] about first probable observation of two-bond scalar<br />
31 P− 1 H coupling constants J across the N−H···O − −P + intramolecular hydrogen bond (H<br />
bond) in the Z forms of four O,O-dialkyl 1-oxoalkanephosphonate hydrazones 1 (R = Me, t-<br />
Bu, CH 2 Ph, Ph; J PH 2.9 ± 0.5 Hz, from 1 H and/or 31 P proton-coupled NMR spectra), investigated<br />
previously by NMR [2]. Our work was subsequently followed by two experimental<br />
findings of similar H-bond-mediated interactions involving a P nucleus in protein-DNA complexes<br />
[3] or D. vulgaris flavodoxin (flavoprotein) [4], supported later by deMon-NMR code<br />
based DFT computations [5] for the models of aforementioned biological systems. In addition,<br />
other related papers were published (see e.g., refs 68-72 in recent review on J-coupling<br />
calculations [6]). In the case of compounds (Z)-1, two possibilities exist for explaining the observed<br />
heteronuclear J PH coupling, namely, the P↔H interaction across the N−H···O − −P +<br />
hydrogen bond ( h2 J) in the formed 6-membered ring, or traditional coupling along a longrange<br />
pathway via the molecular backbone ( 4 J). The best simple method to choose between<br />
these two pathways was a theoretical study. Results of presented DFT/FPT computations suggest<br />
that the foregoing interactions in 1 are in fact due to an across-H-bond J coupling. As the<br />
h2 J PH coupling seems to be a sensitive probe of the H-bond angle and distance [5], an interesting<br />
question of its sign will be discussed in detail in the poster version of this communication.<br />
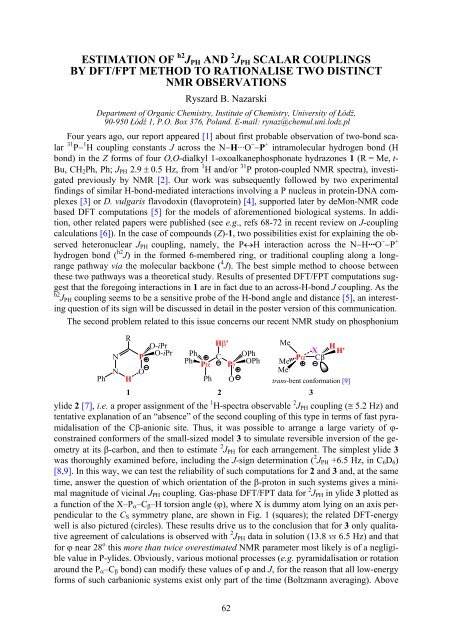
The second problem related to this issue concerns our recent NMR study on phosphonium<br />
Ph<br />
N<br />
N<br />
R<br />
H<br />
P<br />
O<br />
O-iPr<br />
O-iPr<br />
Ph<br />
Ph<br />
Pα<br />
Ph<br />
C<br />
1 2<br />
Hβ'<br />
ylide 2 [7], i.e. a proper assignment of the 1 H-spectra observable 2 J PH coupling (≅ 5.2 Hz) and<br />
tentative explanation of an “absence” of the second coupling of this type in terms of fast pyramidalisation<br />
of the Cβ-anionic site. Thus, it was possible to arrange a large variety of ϕ-<br />
constrained conformers of the small-sized model 3 to simulate reversible inversion of the geometry<br />
at its β-carbon, and then to estimate 2 J PH for each arrangement. The simplest ylide 3<br />
was thoroughly examined before, including the J-sign determination ( 2 J PH +6.5 Hz, in C 6 D 6 )<br />
[8,9]. In this way, we can test the reliability of such computations for 2 and 3 and, at the same<br />
time, answer the question of which orientation of the β-proton in such systems gives a minimal<br />
magnitude of vicinal J PH coupling. Gas-phase DFT/FPT data for 2 J PH in ylide 3 plotted as<br />
a function of the X–P α –C β –H torsion angle (ϕ), where X is dummy atom lying on an axis perpendicular<br />
to the C S symmetry plane, are shown in Fig. 1 (squares); the related DFT-energy<br />
well is also pictured (circles). These results drive us to the conclusion that for 3 only qualitative<br />
agreement of calculations is observed with 2 J PH data in solution (13.8 vs 6.5 Hz) and that<br />
for ϕ near 28 o this more than twice overestimated NMR parameter most likely is of a negligible<br />
value in P-ylides. Obviously, various motional processes (e.g. pyramidalisation or rotation<br />
around the P α –C β bond) can modify these values of ϕ and J, for the reason that all low-energy<br />
forms of such carbanionic systems exist only part of the time (Boltzmann averaging). Above<br />
Pγ<br />
O<br />
OPh<br />
OPh<br />
Me<br />
Me<br />
Me<br />
Pα<br />
X<br />
Cβ<br />
trans-bent conformation [9]<br />
3<br />
H<br />
H'<br />
62