Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
Report No xxxx - Instytut Fizyki JÄ drowej PAN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
T 1 DISPERSION AND SELF-DIFFUSION NMR STUDY<br />
OF WATER MOLECULES IN POLY(ACRYLIC ACID) HYDROGELS<br />
Grzegorz <strong>No</strong>waczyk, Marek Kempka, Stefan Jurga<br />
Institute of Physics, Adam Mickiewicz University, Umultowska 85,PL-61614 Poznań, Poland<br />
A detailed understanding of the structure of hydrogels and the dynamics of molecular<br />
motions of water in gels is very important for biomedical drug delivery processes that use<br />
hydrogels as carriers (1).<br />
One of the most extensively used and studied hydrogels is poly(acrylic acid) gel. Here<br />
we report studies of water molecules in poly(acrylic acid) microgels (carbopol ® 971) by means<br />
of Fast Field Cycling Realaxometry and NMR diffusion methods.<br />
Carbopol ® 971 was provided by <strong>No</strong>veon, Inc. The polymer powder was dispersed in<br />
distillated water with or without surfactants. The polymer content of gels was 0,5 and 1%.<br />
10% NaOH was added to neutralize the sample to pH 3.<br />
T 1 dispersion of water was measured in the frequency range from 10 kHz to 9 MHz at<br />
293 and 281K, whereas the self-diffusion measurements were performed using homemade<br />
spin-echo NMR spectrometer operating at 16,5 MHz at temperature range from 297 to 323K.<br />
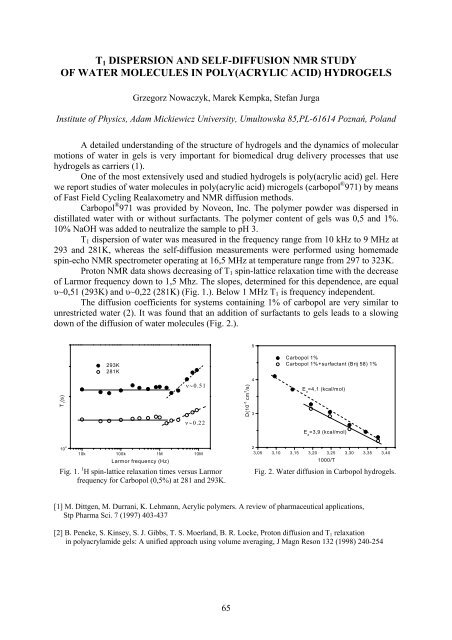
Proton NMR data shows decreasing of T 1 spin-lattice relaxation time with the decrease<br />
of Larmor frequency down to 1,5 Mhz. The slopes, determined for this dependence, are equal<br />
υ~0,51 (293K) and υ~0,22 (281K) (Fig. 1.). Below 1 MHz T 1 is frequency independent.<br />
The diffusion coefficients for systems containing 1% of carbopol are very similar to<br />
unrestricted water (2). It was found that an addition of surfactants to gels leads to a slowing<br />
down of the diffusion of water molecules (Fig. 2.).<br />
5<br />
T 1<br />
(s)<br />
293K<br />
281K<br />
ν∼0.51<br />
10 0 ν∼0.22<br />
10k 100k 1M 10M<br />
Larmor frequency (Hz)<br />
Fig. 1. 1 H spin-lattice relaxation times versus Larmor<br />
frequency for Carbopol (0,5%) at 281 and 293K.<br />
D(10 -5 cm 2 /s)<br />
4<br />
3<br />
Carbopol 1%<br />
Carbopol 1%+surfactant (Brij 58) 1%<br />
E a<br />
=4,1 (kcal/mol)<br />
E a<br />
=3,9 (kcal/mol)<br />
2<br />
3,05 3,10 3,15 3,20 3,25 3,30 3,35 3,40<br />
1000/T<br />
Fig. 2. Water diffusion in Carbopol hydrogels.<br />
[1] M. Dittgen, M. Durrani, K. Lehmann, Acrylic polymers. A review of pharmaceutical applications,<br />
Stp Pharma Sci. 7 (1997) 403-437<br />
[2] B. Peneke, S. Kinsey, S. J. Gibbs, T. S. Moerland, B. R. Locke, Proton diffusion and T 1 relaxation<br />
in polyacrylamide gels: A unified approach using volume averaging, J Magn Reson 132 (1998) 240-254<br />
65