School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Results<br />
5 Results<br />
5.1 Analysis <strong>of</strong> siderophores produced by Pss22d<br />
Under iron-limiting conditions many pseudomonads produce a yellow-greenish,<br />
fluorescent pigment, thus they are classified as “fluorescent pseudomonads”.<br />
These pigments are peptide-type siderophores called pyoverdins. All<br />
pyoverdins share the same dihydroxyquinoline chromophore responsible for<br />
their fluorescence, with an amidically connected small dicarboxylic acid (or its<br />
monoamide). The peptide chain bound to the chromophore carboxyl group<br />
varies. It consists <strong>of</strong> 6-12 amino acids <strong>of</strong>ten including modified amino acids or<br />
D-forms (Fuchs et al., 2001). Approximately 50 different pyoverdins have been<br />
identified so far <strong>and</strong> the type <strong>of</strong> pyoverdin produced can vary even among<br />
strains <strong>of</strong> the same species, for example 3 different pyoverdins have been<br />
characterized for P. aeruginosa (de Chial et al., 2003). In contrast to their<br />
otherwise prominent diversity, all pathovars <strong>of</strong> P. syringae tested so far produce<br />
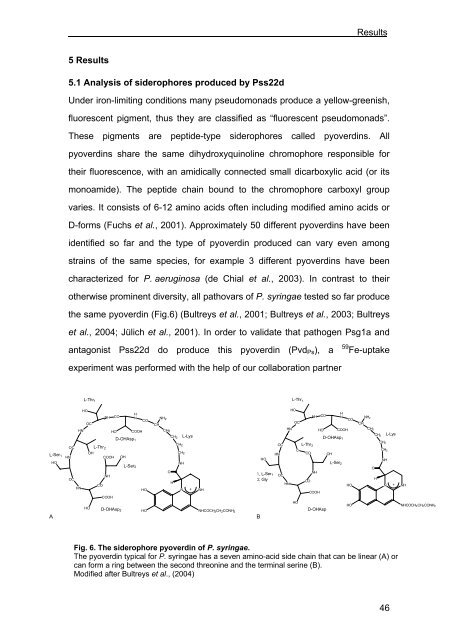
the same pyoverdin (Fig.6) (Bultreys et al., 2001; Bultreys et al., 2003; Bultreys<br />
et al., 2004; Jülich et al., 2001). In order to validate that pathogen Psg1a <strong>and</strong><br />
antagonist Pss22d do produce this pyoverdin (Pvd Ps ), a<br />
59 Fe-uptake<br />
experiment was performed with the help <strong>of</strong> our collaboration partner<br />
HO<br />
L-Thr 1<br />
HO<br />
H<br />
NH CO N<br />
CO<br />
OC<br />
HN<br />
HO COOH<br />
D-OHAsp 1<br />
OC L-Thr 2<br />
OH<br />
HN<br />
COOH OH<br />
L-Ser 2<br />
L-Ser 1<br />
D-OHAsp 2<br />
A<br />
OC<br />
HN<br />
HO<br />
CO<br />
NH<br />
COOH<br />
HO<br />
HO<br />
NH 2<br />
CH<br />
CH2<br />
CH 2 L-Lys<br />
CH 2<br />
CH2<br />
NH<br />
H<br />
N +<br />
O<br />
NH<br />
NHCOCH 2 CH 2 CONH 2<br />
1, L-Ser 1<br />
2, Gly<br />
B<br />
HO<br />
OC<br />
HN<br />
OC<br />
HN<br />
HO<br />
OC<br />
HN<br />
O<br />
HO<br />
L-Thr 1<br />
L-Thr 2<br />
CO<br />
CO<br />
NH<br />
NH<br />
COOH<br />
CO<br />
HO<br />
D-OHAsp<br />
OH<br />
H<br />
N<br />
COOH<br />
D-OHAsp 1<br />
L-Ser 2<br />
CO<br />
HO<br />
HO<br />
NH 2<br />
CH<br />
CH2<br />
CH 2 L-Lys<br />
CH2<br />
CH 2<br />
NH<br />
H<br />
N<br />
O<br />
+<br />
NH<br />
NHCOCH2CH2CONH2<br />
Fig. 6. The siderophore pyoverdin <strong>of</strong> P. syringae.<br />
The pyoverdin typical for P. syringae has a seven amino-acid side chain that can be linear (A) or<br />
can form a ring between the second threonine <strong>and</strong> the terminal serine (B).<br />
Modified after Bultreys et al., (2004)<br />
46