FOUNDATIONS OF QUANTUM MECHANICS
FOUNDATIONS OF QUANTUM MECHANICS
FOUNDATIONS OF QUANTUM MECHANICS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
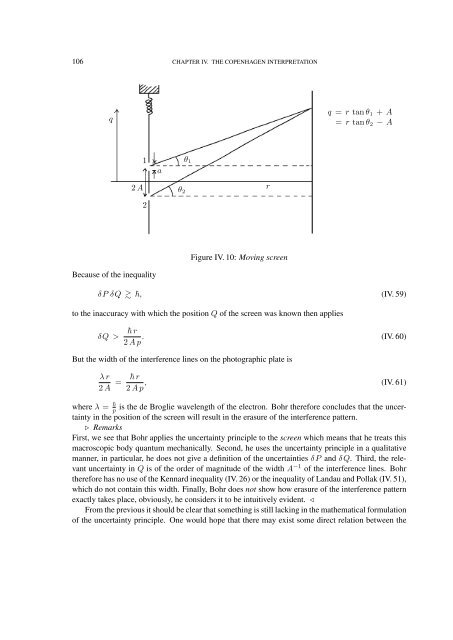
106 CHAPTER IV. THE COPENHAGEN INTERPRETATION<br />
q<br />
q = r tan θ 1 + A<br />
= r tan θ 2 − A<br />
2 A<br />
1<br />
2<br />
a<br />
θ 2<br />
θ 1<br />
r<br />
Figure IV. 10: Moving screen<br />
Because of the inequality<br />
δP δQ , (IV. 59)<br />
to the inaccuracy with which the position Q of the screen was known then applies<br />
δQ ><br />
r . (IV. 60)<br />
2 A p<br />
But the width of the interference lines on the photographic plate is<br />
λ r<br />
2 A = r , (IV. 61)<br />
2 A p<br />
where λ = p<br />
is the de Broglie wavelength of the electron. Bohr therefore concludes that the uncertainty<br />
in the position of the screen will result in the erasure of the interference pattern.<br />
◃ Remarks<br />
First, we see that Bohr applies the uncertainty principle to the screen which means that he treats this<br />
macroscopic body quantum mechanically. Second, he uses the uncertainty principle in a qualitative<br />
manner, in particular, he does not give a definition of the uncertainties δP and δQ. Third, the relevant<br />
uncertainty in Q is of the order of magnitude of the width A −1 of the interference lines. Bohr<br />
therefore has no use of the Kennard inequality (IV. 26) or the inequality of Landau and Pollak (IV. 51),<br />
which do not contain this width. Finally, Bohr does not show how erasure of the interference pattern<br />
exactly takes place, obviously, he considers it to be intuitively evident. ▹<br />
From the previous it should be clear that something is still lacking in the mathematical formulation<br />
of the uncertainty principle. One would hope that there may exist some direct relation between the