Sterlite Industries (India) Limited - Sterlite Industries India Ltd.
Sterlite Industries (India) Limited - Sterlite Industries India Ltd.
Sterlite Industries (India) Limited - Sterlite Industries India Ltd.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
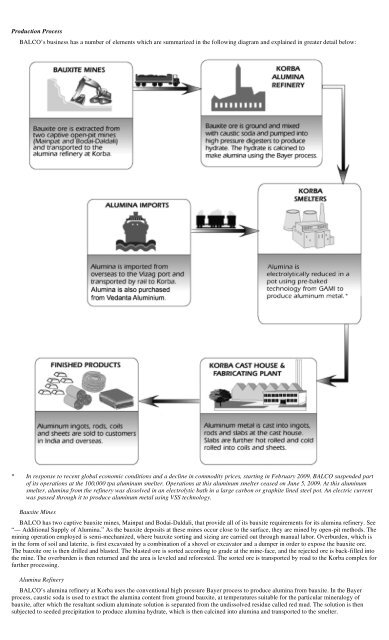
Production Process<br />
BALCO’s business has a number of elements which are summarized in the following diagram and explained in greater detail below:<br />
* In response to recent global economic conditions and a decline in commodity prices, starting in February 2009, BALCO suspended part<br />
of its operations at the 100,000 tpa aluminum smelter. Operations at this aluminum smelter ceased on June 5, 2009. At this aluminum<br />
smelter, alumina from the refinery was dissolved in an electrolytic bath in a large carbon or graphite lined steel pot. An electric current<br />
was passed through it to produce aluminum metal using VSS technology.<br />
Bauxite Mines<br />
BALCO has two captive bauxite mines, Mainpat and Bodai-Daldali, that provide all of its bauxite requirements for its alumina refinery. See<br />
“— Additional Supply of Alumina.” As the bauxite deposits at these mines occur close to the surface, they are mined by open-pit methods. The<br />
mining operation employed is semi-mechanized, where bauxite sorting and sizing are carried out through manual labor. Overburden, which is<br />
in the form of soil and laterite, is first excavated by a combination of a shovel or excavator and a dumper in order to expose the bauxite ore.<br />
The bauxite ore is then drilled and blasted. The blasted ore is sorted according to grade at the mine-face, and the rejected ore is back-filled into<br />
the mine. The overburden is then returned and the area is leveled and reforested. The sorted ore is transported by road to the Korba complex for<br />
further processing.<br />
Alumina Refinery<br />
BALCO’s alumina refinery at Korba uses the conventional high pressure Bayer process to produce alumina from bauxite. In the Bayer<br />
process, caustic soda is used to extract the alumina content from ground bauxite, at temperatures suitable for the particular mineralogy of<br />
bauxite, after which the resultant sodium aluminate solution is separated from the undissolved residue called red mud. The solution is then<br />
subjected to seeded precipitation to produce alumina hydrate, which is then calcined into alumina and transported to the smelter.