GSK Annual Report 2002
GSK Annual Report 2002
GSK Annual Report 2002
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
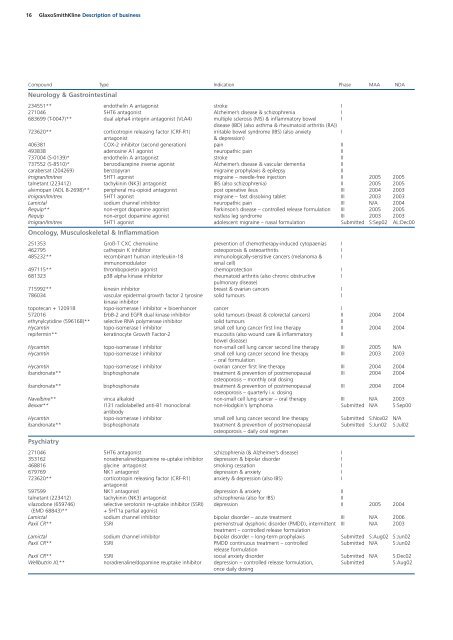
16 GlaxoSmithKline Description of business<br />
Compound Type Indication Phase MAA NDA<br />
Neurology & Gastrointestinal<br />
234551** endothelin A antagonist stroke I<br />
271046 5HT6 antagonist Alzheimer’s disease & schizophrenia I<br />
683699 (T-0047)** dual alpha4 integrin antagonist (VLA4) multiple sclerosis (MS) & inflammatory bowel I<br />
disease (IBD) (also asthma & rheumatoid arthritis (RA))<br />
723620** corticotropin releasing factor (CRF-R1) irritable bowel syndrome (IBS) (also anxiety I<br />
antagonist & depression)<br />
406381 COX-2 inhibitor (second generation) pain II<br />
493838 adenosine A1 agonist neuropathic pain II<br />
737004 (S-0139)* endothelin A antagonist stroke II<br />
737552 (S-8510)* benzodiazepine inverse agonist Alzheimer’s disease & vascular dementia II<br />
carabersat (204269) benzopyran migraine prophylaxis & epilepsy II<br />
Imigran/Imitrex 5HT1 agonist migraine – needle-free injection II 2005 2005<br />
talnetant (223412) tachykinin (NK3) antagonist IBS (also schizophrenia) II 2005 2005<br />
alvimopan (ADL 8-2698)** peripheral mu-opioid antagonist post operative ileus III 2004 2003<br />
Imigran/Imitrex 5HT1 agonist migraine – fast dissolving tablet III 2003 2003<br />
Lamictal sodium channel inhibitor neuropathic pain III N/A 2004<br />
Requip** non-ergot dopamine agonist Parkinson’s disease – controlled release formulation III 2005 2005<br />
Requip non-ergot dopamine agonist restless leg syndrome III 2003 2003<br />
Imigran/Imitrex 5HT1 agonist adolescent migraine – nasal formulation Submitted S:Sep02 AL:Dec00<br />
Oncology, Musculoskeletal & Inflammation<br />
251353 Groß-T CXC chemokine prevention of chemotherapy-induced cytopaenias I<br />
462795 cathepsin K inhibitor osteoporosis & osteoarthritis I<br />
485232** recombinant human interleukin-18 immunologically-sensitive cancers (melanoma & I<br />
immunomodulator renal cell)<br />
497115** thrombopoietin agonist chemoprotection I<br />
681323 p38 alpha kinase inhibitor rheumatoid arthritis (also chronic obstructive I<br />
pulmonary disease)<br />
715992** kinesin inhibitor breast & ovarian cancers I<br />
786034 vascular epidermal growth factor 2 tyrosine solid tumours I<br />
kinase inhibitor<br />
topotecan + 120918 topo-isomerase l inhibitor + bioenhancer cancer I<br />
572016 ErbB-2 and EGFR dual kinase inhibitor solid tumours (breast & colorectal cancers) II 2004 2004<br />
ethynylcytidine (596168)** selective RNA polymerase inhibitor solid tumours II<br />
Hycamtin topo-isomerase I inhibitor small cell lung cancer first line therapy II 2004 2004<br />
repifermin** keratinocyte Growth Factor-2 mucositis (also wound care & inflammatory II<br />
bowel disease)<br />
Hycamtin topo-isomerase I inhibitor non-small cell lung cancer second line therapy III 2005 N/A<br />
Hycamtin topo-isomerase I inhibitor small cell lung cancer second line therapy III 2003 2003<br />
– oral formulation<br />
Hycamtin topo-isomerase I inhibitor ovarian cancer first line therapy III 2004 2004<br />
ibandronate** bisphosphonate treatment & prevention of postmenopausal III 2004 2004<br />
osteoporosis – monthly oral dosing<br />
ibandronate** bisphosphonate treatment & prevention of postmenopausal III 2004 2004<br />
osteoporosis – quarterly i.v. dosing<br />
Navelbine** vinca alkaloid non-small cell lung cancer – oral therapy III N/A 2003<br />
Bexxar** I131 radiolabelled anti-B1 monoclonal non-Hodgkin's lymphoma Submitted N/A S:Sep00<br />
antibody<br />
Hycamtin topo-isomerase I inhibitor small cell lung cancer second line therapy Submitted S:Nov02 N/A<br />
ibandronate** bisphosphonate treatment & prevention of postmenopausal Submitted S:Jun02 S:Jul02<br />
osteoporosis – daily oral regimen<br />
Psychiatry<br />
271046 5HT6 antagonist schizophrenia (& Alzheimer’s disease) I<br />
353162 noradrenaline/dopamine re-uptake inhibitor depression & bipolar disorder I<br />
468816 glycine antagonist smoking cessation I<br />
679769 NK1 antagonist depression & anxiety I<br />
723620** corticotropin releasing factor (CRF-R1) anxiety & depression (also IBS) I<br />
antagonist<br />
597599 NK1 antagonist depression & anxiety II<br />
talnetant (223412) tachykinin (NK3) antagonist schizophrenia (also for IBS) II<br />
vilazodone (659746) selective serotonin re-uptake inhibitor (SSRI) depression II 2005 2004<br />
(EMD 68843)** + 5HT1a partial agonist<br />
Lamictal sodium channel inhibitor bipolar disorder – acute treatment III N/A 2006<br />
Paxil CR** SSRI premenstrual dysphoric disorder (PMDD), intermittent III N/A 2003<br />
treatment – controlled release formulation<br />
Lamictal sodium channel inhibitor bipolar disorder – long-term prophylaxis Submitted S:Aug02 S:Jun02<br />
Paxil CR** SSRI PMDD continuous treatment – controlled Submitted N/A S:Jun02<br />
release formulation<br />
Paxil CR** SSRI social anxiety disorder Submitted N/A S:Dec02<br />
Wellbutrin XL** noradrenaline/dopamine reuptake inhibitor depression – controlled release formulation, Submitted S:Aug02<br />
once daily dosing