Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
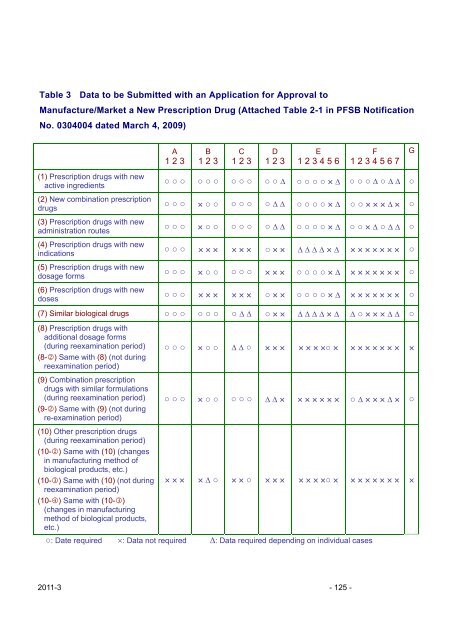
Table 3 Data to be Submitted with an Application for Approval to<br />
Manufacture/Market a New Prescription Drug (Attached Table 2-1 <strong>in</strong> PFSB Notification<br />
No. 0304004 dated March 4, 2009)<br />
(1) Prescription drugs with new<br />
active <strong>in</strong>gredients<br />
(2) New comb<strong>in</strong>ation prescription<br />
drugs<br />
(3) Prescription drugs with new<br />
adm<strong>in</strong>istration routes<br />
(4) Prescription drugs with new<br />
<strong>in</strong>dications<br />
(5) Prescription drugs with new<br />
dosage forms<br />
(6) Prescription drugs with new<br />
doses<br />
A<br />
1 2 3<br />
B<br />
1 2 3<br />
C<br />
1 2 3<br />
D<br />
1 2 3<br />
E<br />
1 2 3 4 5 6<br />
○: Date required ×: Data not required ∆: Data required depend<strong>in</strong>g on <strong>in</strong>dividual cases<br />
2011-3 - 125 -<br />
F<br />
1 2 3 4 5 6 7<br />
○ ○ ○ ○ ○ ○ ○ ○ ○ ○ ○ ∆ ○ ○ ○ ○ × ∆ ○ ○ ○ ∆ ○ ∆ ∆ ○<br />
○ ○ ○ × ○ ○ ○ ○ ○ ○ ∆ ∆ ○ ○ ○ ○ × ∆ ○ ○ × × × ∆ × ○<br />
○ ○ ○ × ○ ○ ○ ○ ○ ○ ∆ ∆ ○ ○ ○ ○ × ∆ ○ ○ × ∆ ○ ∆ ∆ ○<br />
○ ○ ○ × × × × × × ○ × × ∆ ∆ ∆ ∆ × ∆ × × × × × × × ○<br />
○ ○ ○ × ○ ○ ○ ○ ○ × × × ○ ○ ○ ○ × ∆ × × × × × × × ○<br />
○ ○ ○ × × × × × × ○ × × ○ ○ ○ ○ × ∆ × × × × × × × ○<br />
(7) Similar biological drugs ○ ○ ○ ○ ○ ○ ○ ∆ ∆ ○ × × ∆ ∆ ∆ ∆ × ∆ ∆ ○ × × × ∆ ∆ ○<br />
(8) Prescription drugs with<br />
additional dosage forms<br />
(dur<strong>in</strong>g reexam<strong>in</strong>ation period)<br />
(8-2) Same with (8) (not dur<strong>in</strong>g<br />
reexam<strong>in</strong>ation period)<br />
(9) Comb<strong>in</strong>ation prescription<br />
drugs with similar formulations<br />
(dur<strong>in</strong>g reexam<strong>in</strong>ation period)<br />
(9-2) Same with (9) (not dur<strong>in</strong>g<br />
re-exam<strong>in</strong>ation period)<br />
(10) Other prescription drugs<br />
(dur<strong>in</strong>g reexam<strong>in</strong>ation period)<br />
(10-2) Same with (10) (changes<br />
<strong>in</strong> manufactur<strong>in</strong>g method of<br />
biological products, etc.)<br />
(10-3) Same with (10) (not dur<strong>in</strong>g<br />
reexam<strong>in</strong>ation period)<br />
(10-4) Same with (10-3)<br />
(changes <strong>in</strong> manufactur<strong>in</strong>g<br />
method of biological products,<br />
etc.)<br />
○ ○ ○ × ○ ○ ∆ ∆ ○ × × × × × × ×○ × × × × × × × × ×<br />
○ ○ ○ × ○ ○ ○ ○ ○ ∆ ∆ × × × × × × × ○ ∆ × × × ∆ × ○<br />
× × × × ∆ ○ × × ○ × × × × × × ×○ × × × × × × × × ×<br />
G