Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
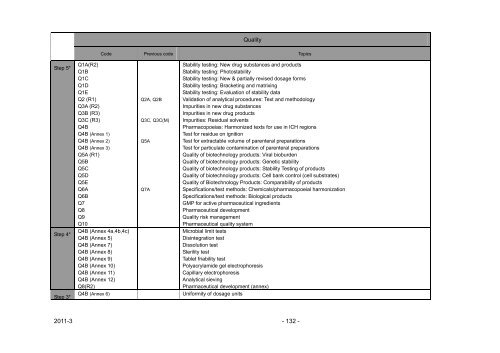
Step 5* Q1A(R2)<br />
Q1B<br />
Q1C<br />
Q1D<br />
Q1E<br />
Q2 (R1)<br />
Q3A (R2)<br />
Q3B (R3)<br />
Q3C (R3)<br />
Q4B<br />
Q4B (Annex 1)<br />
Q4B (Annex 2)<br />
Q4B (Annex 3)<br />
Q5A (R1)<br />
Q5B<br />
Q5C<br />
Q5D<br />
Q5E<br />
Q6A<br />
Q6B<br />
Q7<br />
Q8<br />
Q9<br />
Q10<br />
Q4B (Annex 4a,4b,4c)<br />
Step 4*<br />
Q4B (Annex 5)<br />
Q4B (Annex 7)<br />
Q4B (Annex 8)<br />
Q4B (Annex 9)<br />
Q4B (Annex 10)<br />
Q4B (Annex 11)<br />
Q4B (Annex 12)<br />
Q8(R2)<br />
Step 3*<br />
Quality<br />
Code Previous code Topics<br />
Q2A, Q2B<br />
Q3C, Q3C(M)<br />
Q5A<br />
Q7A<br />
Q4B (Annex 6) Uniformity of dosage units<br />
Stability test<strong>in</strong>g: New drug substances <strong>and</strong> products<br />
Stability test<strong>in</strong>g: Photostability<br />
Stability test<strong>in</strong>g: New & partially revised dosage forms<br />
Stability test<strong>in</strong>g: Bracket<strong>in</strong>g <strong>and</strong> matrix<strong>in</strong>g<br />
Stability test<strong>in</strong>g: Evaluation of stability data<br />
Validation of analytical procedures: Text <strong>and</strong> methodology<br />
Impurities <strong>in</strong> new drug substances<br />
Impurities <strong>in</strong> new drug products<br />
Impurities: Residual solvents<br />
Pharmacopoeias: Harmonized texts for use <strong>in</strong> ICH regions<br />
Test for residue on ignition<br />
Test for extractable volume of parenteral preparations<br />
Test for particulate contam<strong>in</strong>ation of parenteral preparations<br />
Quality of biotechnology products: Viral bioburden<br />
Quality of biotechnology products: Genetic stability<br />
Quality of biotechnology products: Stability Test<strong>in</strong>g of products<br />
Quality of biotechnology products: Cell bank control (cell substrates)<br />
Quality of Biotechnology Products: Comparability of products<br />
Specifications/test methods: Chemicals/pharmacopoeial harmonization<br />
Specifications/test methods: Biological products<br />
GMP for active pharmaceutical <strong>in</strong>gredients<br />
<strong>Pharmaceutical</strong> development<br />
Quality risk management<br />
<strong>Pharmaceutical</strong> quality system<br />
Microbial limit tests<br />
Dis<strong>in</strong>tegration test<br />
Dissolution test<br />
Sterility test<br />
Tablet friability test<br />
Polyacrylamide gel electrophoresis<br />
Capillary electrophoresis<br />
Analytical siev<strong>in</strong>g<br />
<strong>Pharmaceutical</strong> development (annex)<br />
2011-3 - 132 -