Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
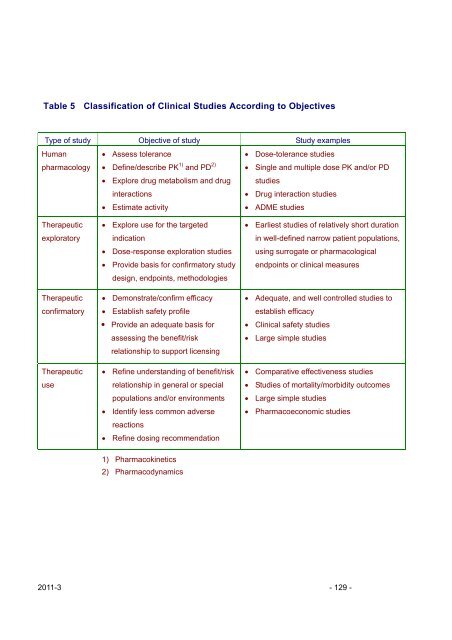
Table 5 Classification of Cl<strong>in</strong>ical Studies Accord<strong>in</strong>g to Objectives<br />
Type of study Objective of study Study examples<br />
Human • Assess tolerance<br />
pharmacology • Def<strong>in</strong>e/describe PK 1) <strong>and</strong> PD 2)<br />
• Dose-tolerance studies<br />
• S<strong>in</strong>gle <strong>and</strong> multiple dose PK <strong>and</strong>/or PD<br />
• Explore drug metabolism <strong>and</strong> drug studies<br />
<strong>in</strong>teractions<br />
• Drug <strong>in</strong>teraction studies<br />
• Estimate activity<br />
• ADME studies<br />
Therapeutic<br />
exploratory<br />
Therapeutic<br />
confirmatory<br />
Therapeutic<br />
use<br />
• Explore use for the targeted<br />
<strong>in</strong>dication<br />
• Dose-response exploration studies<br />
• Provide basis for confirmatory study<br />
design, endpo<strong>in</strong>ts, methodologies<br />
• Demonstrate/confirm efficacy<br />
• Establish safety profile<br />
� Provide an adequate basis for<br />
assess<strong>in</strong>g the benefit/risk<br />
relationship to support licens<strong>in</strong>g<br />
• Ref<strong>in</strong>e underst<strong>and</strong><strong>in</strong>g of benefit/risk<br />
relationship <strong>in</strong> general or special<br />
populations <strong>and</strong>/or environments<br />
• Identify less common adverse<br />
reactions<br />
• Ref<strong>in</strong>e dos<strong>in</strong>g recommendation<br />
1) Pharmacok<strong>in</strong>etics<br />
2) Pharmacodynamics<br />
• Earliest studies of relatively short duration<br />
<strong>in</strong> well-def<strong>in</strong>ed narrow patient populations,<br />
us<strong>in</strong>g surrogate or pharmacological<br />
endpo<strong>in</strong>ts or cl<strong>in</strong>ical measures<br />
• Adequate, <strong>and</strong> well controlled studies to<br />
establish efficacy<br />
• Cl<strong>in</strong>ical safety studies<br />
• Large simple studies<br />
• Comparative effectiveness studies<br />
• Studies of mortality/morbidity outcomes<br />
• Large simple studies<br />
• Pharmacoeconomic studies<br />
2011-3 - 129 -