Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
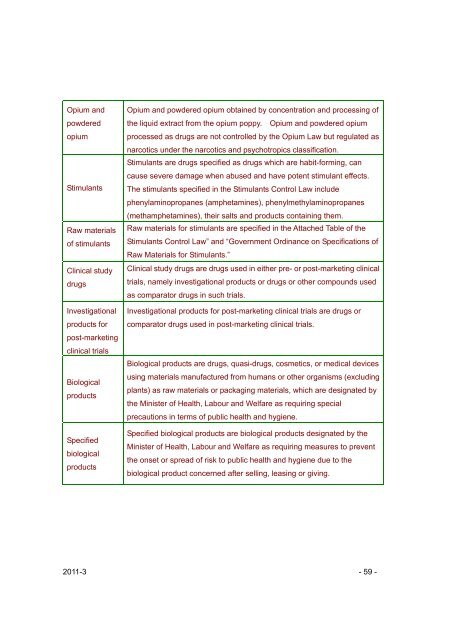
Opium <strong>and</strong><br />
powdered<br />
opium<br />
Stimulants<br />
Raw materials<br />
of stimulants<br />
Cl<strong>in</strong>ical study<br />
drugs<br />
Investigational<br />
products for<br />
post-market<strong>in</strong>g<br />
cl<strong>in</strong>ical trials<br />
Biological<br />
products<br />
Specified<br />
biological<br />
products<br />
Opium <strong>and</strong> powdered opium obta<strong>in</strong>ed by concentration <strong>and</strong> process<strong>in</strong>g of<br />
the liquid extract from the opium poppy. Opium <strong>and</strong> powdered opium<br />
processed as drugs are not controlled by the Opium Law but regulated as<br />
narcotics under the narcotics <strong>and</strong> psychotropics classification.<br />
Stimulants are drugs specified as drugs which are habit-form<strong>in</strong>g, can<br />
cause severe damage when abused <strong>and</strong> have potent stimulant effects.<br />
The stimulants specified <strong>in</strong> the Stimulants Control Law <strong>in</strong>clude<br />
phenylam<strong>in</strong>opropanes (amphetam<strong>in</strong>es), phenylmethylam<strong>in</strong>opropanes<br />
(methamphetam<strong>in</strong>es), their salts <strong>and</strong> products conta<strong>in</strong><strong>in</strong>g them.<br />
Raw materials for stimulants are specified <strong>in</strong> the Attached Table of the<br />
Stimulants Control Law” <strong>and</strong> “Government Ord<strong>in</strong>ance on Specifications of<br />
Raw Materials for Stimulants.”<br />
Cl<strong>in</strong>ical study drugs are drugs used <strong>in</strong> either pre- or post-market<strong>in</strong>g cl<strong>in</strong>ical<br />
trials, namely <strong>in</strong>vestigational products or drugs or other compounds used<br />
as comparator drugs <strong>in</strong> such trials.<br />
Investigational products for post-market<strong>in</strong>g cl<strong>in</strong>ical trials are drugs or<br />
comparator drugs used <strong>in</strong> post-market<strong>in</strong>g cl<strong>in</strong>ical trials.<br />
Biological products are drugs, quasi-drugs, cosmetics, or medical devices<br />
us<strong>in</strong>g materials manufactured from humans or other organisms (exclud<strong>in</strong>g<br />
plants) as raw materials or packag<strong>in</strong>g materials, which are designated by<br />
the M<strong>in</strong>ister of Health, Labour <strong>and</strong> Welfare as requir<strong>in</strong>g special<br />
precautions <strong>in</strong> terms of public health <strong>and</strong> hygiene.<br />
Specified biological products are biological products designated by the<br />
M<strong>in</strong>ister of Health, Labour <strong>and</strong> Welfare as requir<strong>in</strong>g measures to prevent<br />
the onset or spread of risk to public health <strong>and</strong> hygiene due to the<br />
biological product concerned after sell<strong>in</strong>g, leas<strong>in</strong>g or giv<strong>in</strong>g.<br />
2011-3 - 59 -