Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Step 2*<br />
Step 1*<br />
Step 5*<br />
Step 4*<br />
Step 3*<br />
Step 2*<br />
Step 1*<br />
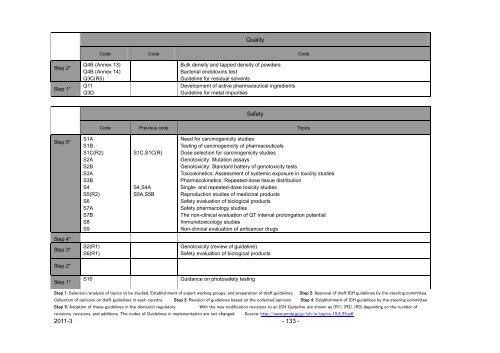
Quality<br />
Code Code Code<br />
Q4B (Annex 13)<br />
Q4B (Annex 14)<br />
Q3C(R5)<br />
Q11<br />
Q3D<br />
S1A<br />
S1B<br />
S1C(R2)<br />
S2A<br />
S2B<br />
S3A<br />
S3B<br />
S4<br />
S5(R2)<br />
S6<br />
S7A<br />
S7B<br />
S8<br />
S9<br />
S2(R1)<br />
S6(R1)<br />
Bulk density <strong>and</strong> tapped density of powders<br />
Bacterial endotox<strong>in</strong>s test<br />
Guidel<strong>in</strong>e for residual solvents<br />
Development of active pharmaceutical <strong>in</strong>gredients<br />
Guidel<strong>in</strong>e for metal impurities<br />
Safety<br />
Code Previous code Topics<br />
S1C,S1C(R)<br />
S4,S4A<br />
S5A,S5B<br />
Need for carc<strong>in</strong>ogenicity studies<br />
Test<strong>in</strong>g of carc<strong>in</strong>ogenicity of pharmaceuticals<br />
Dose selection for carc<strong>in</strong>ogenicity studies<br />
Genotoxicity: Mutation assays<br />
Genotoxicity: St<strong>and</strong>ard battery of genotoxicity tests<br />
Toxicok<strong>in</strong>etics: Assessment of systemic exposure <strong>in</strong> toxicity studies<br />
Pharmacok<strong>in</strong>etics: Repeated-dose tissue distribution<br />
S<strong>in</strong>gle- <strong>and</strong> repeated-dose toxicity studies<br />
Reproduction studies of medic<strong>in</strong>al products<br />
Safety evaluation of biological products<br />
Safety pharmacology studies<br />
The non-cl<strong>in</strong>ical evaluation of QT <strong>in</strong>terval prolongation potential<br />
Immunotoxicology studies<br />
Non-cl<strong>in</strong>ical evaluation of anticancer drugs<br />
Genotoxicity (review of guidel<strong>in</strong>e)<br />
Safety evaluation of biological products<br />
S10 Guidance on photosafety test<strong>in</strong>g<br />
Step 1: Selection/analysis of topics to be studied. Establishment of expert work<strong>in</strong>g groups, <strong>and</strong> preparation of draft guidel<strong>in</strong>es Step 2: Approval of draft ICH guidel<strong>in</strong>es by the steer<strong>in</strong>g committee.<br />
Collection of op<strong>in</strong>ions on draft guidel<strong>in</strong>es <strong>in</strong> each country Step 3: Revision of guidel<strong>in</strong>es based on the collected op<strong>in</strong>ions Step 4: Establishment of ICH guidel<strong>in</strong>es by the steer<strong>in</strong>g committee<br />
Step 5: Adoption of these guidel<strong>in</strong>es <strong>in</strong> the domestic regulatory With the new codification revisions to an ICH Guidel<strong>in</strong>e are shown as (R1), (R2), (R3) depend<strong>in</strong>g on the number of<br />
revisions, revisions, <strong>and</strong> additions. The codes of Guidel<strong>in</strong>es <strong>in</strong> implementation are not changed. Source: http://www.pmda.go.jp/ich/w/topics_10_6_25.pdf<br />
2011-3 - 133 -