Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Pharmaceutical Administration and Regulations in Japan - Nihs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
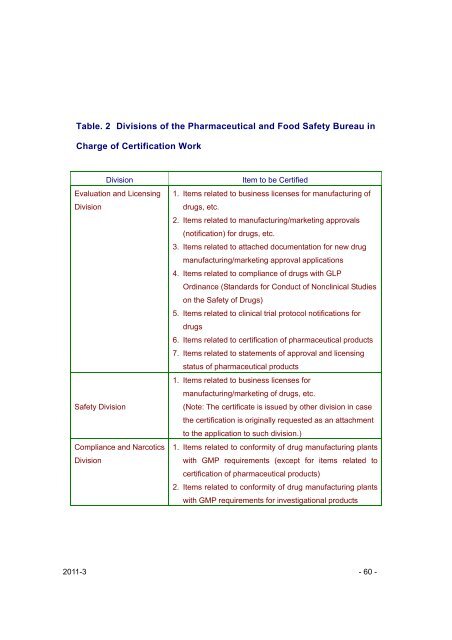
Table. 2 Divisions of the <strong>Pharmaceutical</strong> <strong>and</strong> Food Safety Bureau <strong>in</strong><br />
Charge of Certification Work<br />
Division Item to be Certified<br />
Evaluation <strong>and</strong> Licens<strong>in</strong>g 1. Items related to bus<strong>in</strong>ess licenses for manufactur<strong>in</strong>g of<br />
Division<br />
drugs, etc.<br />
2. Items related to manufactur<strong>in</strong>g/market<strong>in</strong>g approvals<br />
(notification) for drugs, etc.<br />
3. Items related to attached documentation for new drug<br />
manufactur<strong>in</strong>g/market<strong>in</strong>g approval applications<br />
4. Items related to compliance of drugs with GLP<br />
Ord<strong>in</strong>ance (St<strong>and</strong>ards for Conduct of Noncl<strong>in</strong>ical Studies<br />
on the Safety of Drugs)<br />
5. Items related to cl<strong>in</strong>ical trial protocol notifications for<br />
drugs<br />
6. Items related to certification of pharmaceutical products<br />
7. Items related to statements of approval <strong>and</strong> licens<strong>in</strong>g<br />
status of pharmaceutical products<br />
Safety Division<br />
Compliance <strong>and</strong> Narcotics<br />
Division<br />
1. Items related to bus<strong>in</strong>ess licenses for<br />
manufactur<strong>in</strong>g/market<strong>in</strong>g of drugs, etc.<br />
(Note: The certificate is issued by other division <strong>in</strong> case<br />
the certification is orig<strong>in</strong>ally requested as an attachment<br />
to the application to such division.)<br />
1. Items related to conformity of drug manufactur<strong>in</strong>g plants<br />
with GMP requirements (except for items related to<br />
certification of pharmaceutical products)<br />
2. Items related to conformity of drug manufactur<strong>in</strong>g plants<br />
with GMP requirements for <strong>in</strong>vestigational products<br />
2011-3 - 60 -