Development of hot-melt extrusion as a novel technique for the ...
Development of hot-melt extrusion as a novel technique for the ...
Development of hot-melt extrusion as a novel technique for the ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
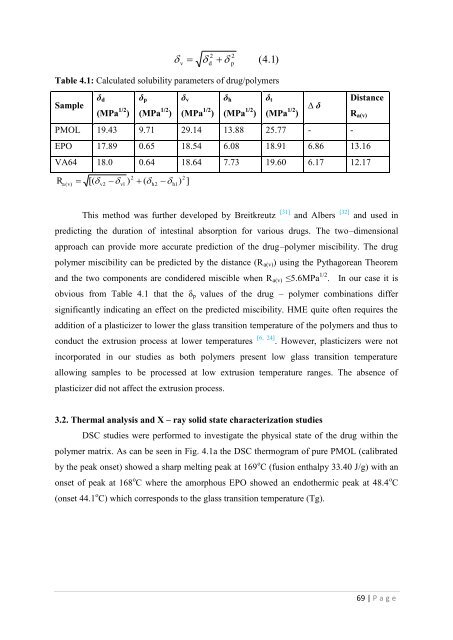
v2d 2p(4.1)Table 4.1: Calculated solubility parameters <strong>of</strong> drug/polymersSample d(MPa 1/2 ) p(MPa 1/2 ) v(MPa 1/2 ) h(MPa 1/2 ) t(MPa 1/2 )PMOL 19.43 9.71 29.14 13.88 25.77 - -∆ DistanceR a(v)EPO 17.89 0.65 18.54 6.08 18.91 6.86 13.16VA64 18.0 0.64 18.64 7.73 19.60 6.17 12.17Ra[( ) ( )2( v)v2v1h2h12]This method w<strong>as</strong> fur<strong>the</strong>r developed by Breitkreutz [31] and Albers [32] and used inpredicting <strong>the</strong> duration <strong>of</strong> intestinal absorption <strong>for</strong> various drugs. The two–dimensionalapproach can provide more accurate prediction <strong>of</strong> <strong>the</strong> drug–polymer miscibility. The drugpolymer miscibility can be predicted by <strong>the</strong> distance (R a(v) ) using <strong>the</strong> Pythagorean Theoremand <strong>the</strong> two components are condidered miscible when R a(v) ≤5.6MPa 1/2 . In our c<strong>as</strong>e it isobvious from Table 4.1 that <strong>the</strong> p values <strong>of</strong> <strong>the</strong> drug – polymer combinations differsignificantly indicating an effect on <strong>the</strong> predicted miscibility. HME quite <strong>of</strong>ten requires <strong>the</strong>addition <strong>of</strong> a pl<strong>as</strong>ticizer to lower <strong>the</strong> gl<strong>as</strong>s transition temperature <strong>of</strong> <strong>the</strong> polymers and thus toconduct <strong>the</strong> <strong>extrusion</strong> process at lower temperatures [6, 24] . However, pl<strong>as</strong>ticizers were notincorporated in our studies <strong>as</strong> both polymers present low gl<strong>as</strong>s transition temperatureallowing samples to be processed at low <strong>extrusion</strong> temperature ranges. The absence <strong>of</strong>pl<strong>as</strong>ticizer did not affect <strong>the</strong> <strong>extrusion</strong> process.3.2. Thermal analysis and X – ray solid state characterization studiesDSC studies were per<strong>for</strong>med to investigate <strong>the</strong> physical state <strong>of</strong> <strong>the</strong> drug within <strong>the</strong>polymer matrix. As can be seen in Fig. 4.1a <strong>the</strong> DSC <strong>the</strong>rmogram <strong>of</strong> pure PMOL (calibratedby <strong>the</strong> peak onset) showed a sharp <strong>melt</strong>ing peak at 169 o C (fusion enthalpy 33.40 J/g) with anonset <strong>of</strong> peak at 168 o C where <strong>the</strong> amorphous EPO showed an endo<strong>the</strong>rmic peak at 48.4 o C(onset 44.1 o C) which corresponds to <strong>the</strong> gl<strong>as</strong>s transition temperature (Tg).69 | P a g e