H e m a t o lo g y E d u c a t io n - European Hematology Association
H e m a t o lo g y E d u c a t io n - European Hematology Association
H e m a t o lo g y E d u c a t io n - European Hematology Association
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
16 th Congress of the <strong>European</strong> Hemato<strong>lo</strong>gy Associat<strong>io</strong>n<br />
proliferative capacity of the HSCs. 7,8 It has been proposed<br />
that a high repopulat<strong>io</strong>n potential of HSCs is associated<br />
with s<strong>lo</strong>w turnover, and that proliferative quiescence protects<br />
these cells against mutagenic stress and prevents<br />
their exhaust<strong>io</strong>n. 20–22 S<strong>lo</strong>w cycling was confirmed by<br />
analysis of phenotypically characterized primitive<br />
cells. 7,23,24 Seventy percent of most primitive HSCs were<br />
found to be in the G 0 stage of the cell cycle, in contrast to<br />
less than 10% in more differentiated progenitor cells. 7<br />
The concept of dormancy has been discussed for several<br />
decades. For example, it has been shown that the<br />
most primitive HSCs survive a very high dose of the Sphase<br />
specific cytotoxic drug 5-fluorouracil (5-FU) 25 and<br />
that cytokines can be used to increase sensitivity of<br />
HSCs towards 5-FU. 26 However, recently this concept<br />
was proven more directly, when two laboratories have<br />
combined label-retaining assays, f<strong>lo</strong>w cytometry, and<br />
mathematical modeling to demonstrate that less than<br />
30% of HSCs are contained in a dormant state. 7,8 In<br />
these studies, cells were labeled in vivo by traceable<br />
markers that upon cellular divis<strong>io</strong>n were diluted and<br />
became undetectable. Cells that retained the label after<br />
a pro<strong>lo</strong>nged per<strong>io</strong>d of time represented s<strong>lo</strong>wly cycling<br />
cells. Measuring percentages of these label-retaining<br />
cells (LRCs) within the stem cell populat<strong>io</strong>n at different<br />
time points al<strong>lo</strong>wed the authors to deduce the cell<br />
cycling history. Mathematical modeling of these data<br />
demonstrated that the stem cell pool was constituted by<br />
two cell populat<strong>io</strong>ns with different cycling times: “dormant”<br />
HSCs that divided just once in approximately 170<br />
days and “homeostatic” HSCs, which divided every<br />
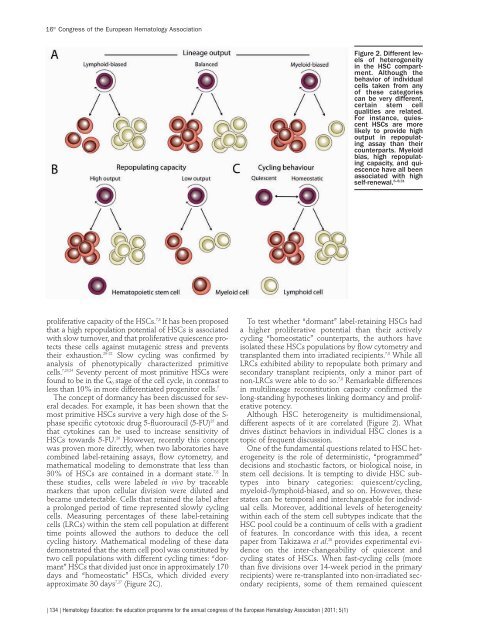
approximate 30 days 7,27 (Figure 2C).<br />
To test whether “dormant” label-retaining HSCs had<br />
a higher proliferative potential than their actively<br />
cycling “homeostatic” counterparts, the authors have<br />
isolated these HSCs populat<strong>io</strong>ns by f<strong>lo</strong>w cytometry and<br />
transplanted them into irradiated recipients. 7,8 While all<br />
LRCs exhibited ability to repopulate both primary and<br />
secondary transplant recipients, only a minor part of<br />
non-LRCs were able to do so. 7,8 Remarkable differences<br />
in multilineage reconstitut<strong>io</strong>n capacity confirmed the<br />
<strong>lo</strong>ng-standing hypotheses linking dormancy and proliferative<br />
potency.<br />
Although HSC heterogeneity is multidimens<strong>io</strong>nal,<br />
different aspects of it are correlated (Figure 2). What<br />
drives distinct behav<strong>io</strong>rs in individual HSC c<strong>lo</strong>nes is a<br />
topic of frequent discuss<strong>io</strong>n.<br />
One of the fundamental quest<strong>io</strong>ns related to HSC heterogeneity<br />
is the role of deterministic, “programmed”<br />
decis<strong>io</strong>ns and stochastic factors, or b<strong>io</strong><strong>lo</strong>gical noise, in<br />
stem cell decis<strong>io</strong>ns. It is tempting to divide HSC subtypes<br />
into binary categories: quiescent/cycling,<br />
mye<strong>lo</strong>id-/lymphoid-biased, and so on. However, these<br />
states can be temporal and interchangeable for individual<br />
cells. Moreover, addit<strong>io</strong>nal levels of heterogeneity<br />
within each of the stem cell subtypes indicate that the<br />
HSC pool could be a continuum of cells with a gradient<br />
of features. In concordance with this idea, a recent<br />
paper from Takizawa et al. 28 provides experimental evidence<br />
on the inter-changeability of quiescent and<br />
cycling states of HSCs. When fast-cycling cells (more<br />
than five divis<strong>io</strong>ns over 14-week per<strong>io</strong>d in the primary<br />
recipients) were re-transplanted into non-irradiated secondary<br />
recipients, some of them remained quiescent<br />
| 134 | Hemato<strong>lo</strong>gy Educat<strong>io</strong>n: the educat<strong>io</strong>n programme for the annual congress of the <strong>European</strong> Hemato<strong>lo</strong>gy Associat<strong>io</strong>n | 2011; 5(1)<br />
Figure 2. Different levels<br />
of heterogeneity<br />
in the HSC compartment.<br />
Although the<br />
behav<strong>io</strong>r of individual<br />
cells taken from any<br />
of these categories<br />
can be very different,<br />
certain stem cell<br />
qualities are related.<br />
For instance, quiescent<br />
HSCs are more<br />
likely to provide high<br />
output in repopulating<br />
assay than their<br />
counterparts. Mye<strong>lo</strong>id<br />
bias, high repopulating<br />
capacity, and quiescence<br />
have all been<br />
associated with high<br />
self-renewal. 6–8,31