H e m a t o lo g y E d u c a t io n - European Hematology Association
H e m a t o lo g y E d u c a t io n - European Hematology Association
H e m a t o lo g y E d u c a t io n - European Hematology Association
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
leed. These issues are especially important from an<br />
economical and societal perspective. In clinical practice,<br />
most prophylactic regimens are tai<strong>lo</strong>red to the need of<br />
the individual patient. Methods to deve<strong>lo</strong>p individual<br />
tai<strong>lo</strong>red regimens have included individual pharmacokinetic<br />
data and computer simulated dose level and interval<br />
to achieve a predetermined trough level. 20,21 In both<br />
hemophilia A and B, this has been accomplished with<br />
verificat<strong>io</strong>n of theoretical data through measurement of<br />
actual FVIII/IX levels. Decreasing intervals between prophylactic<br />
doses from 2–3 infus<strong>io</strong>ns weekly to every<br />
other day theoretically reduces average FVIII consumpt<strong>io</strong>n<br />
by 43% with maintained or increased trough FVIII<br />
levels, while daily dosing would reduce mean FVIII<br />
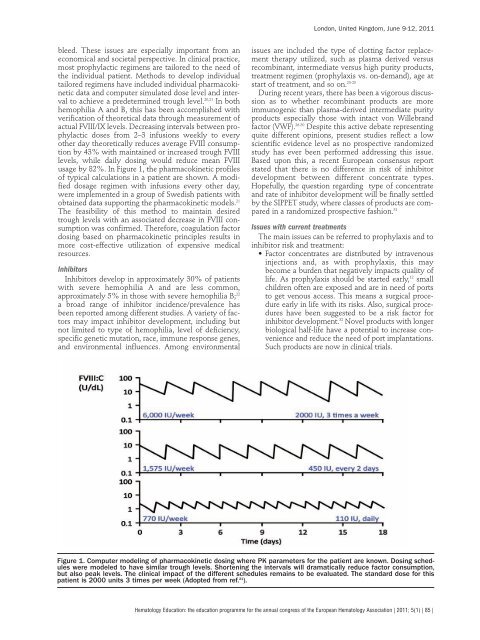
usage by 82%. In Figure 1, the pharmacokinetic profiles<br />
of typical calculat<strong>io</strong>ns in a patient are shown. A modified<br />
dosage regimen with infus<strong>io</strong>ns every other day,<br />
were implemented in a group of Swedish patients with<br />
obtained data supporting the pharmacokinetic models. 21<br />
The feasibility of this method to maintain desired<br />
trough levels with an associated decrease in FVIII consumpt<strong>io</strong>n<br />
was confirmed. Therefore, coagulat<strong>io</strong>n factor<br />
dosing based on pharmacokinetic principles results in<br />
more cost-effective utilizat<strong>io</strong>n of expensive medical<br />
resources.<br />
Inhibitors<br />
Inhibitors deve<strong>lo</strong>p in approximately 30% of patients<br />
with severe hemophilia A and are less common,<br />
approximately 5% in those with severe hemophilia B; 22<br />
a broad range of inhibitor incidence/prevalence has<br />
been reported among different studies. A variety of factors<br />
may impact inhibitor deve<strong>lo</strong>pment, including but<br />
not limited to type of hemophilia, level of deficiency,<br />
specific genetic mutat<strong>io</strong>n, race, immune response genes,<br />
and environmental influences. Among environmental<br />
London, United Kingdom, June 9-12, 2011<br />
issues are included the type of c<strong>lo</strong>tting factor replacement<br />
therapy utilized, such as plasma derived versus<br />
recombinant, intermediate versus high purity products,<br />
treatment regimen (prophylaxis vs. on-demand), age at<br />
start of treatment, and so on. 23-25<br />
During recent years, there has been a vigorous discuss<strong>io</strong>n<br />
as to whether recombinant products are more<br />
immunogenic than plasma-derived intermediate purity<br />
products especially those with intact von Willebrand<br />
factor (VWF). 26-30 Despite this active debate representing<br />
quite different opin<strong>io</strong>ns, present studies reflect a <strong>lo</strong>w<br />
scientific evidence level as no prospective randomized<br />
study has ever been performed addressing this issue.<br />
Based upon this, a recent <strong>European</strong> consensus report<br />
stated that there is no difference in risk of inhibitor<br />
deve<strong>lo</strong>pment between different concentrate types.<br />
Hopefully, the quest<strong>io</strong>n regarding type of concentrate<br />
and rate of inhibitor deve<strong>lo</strong>pment will be finally settled<br />
by the SIPPET study, where classes of products are compared<br />
in a randomized prospective fash<strong>io</strong>n. 31<br />
Issues with current treatments<br />
The main issues can be referred to prophylaxis and to<br />
inhibitor risk and treatment:<br />
• Factor concentrates are distributed by intravenous<br />
inject<strong>io</strong>ns and, as with prophylaxis, this may<br />
become a burden that negatively impacts quality of<br />
life. As prophylaxis should be started early, 12 small<br />
children often are exposed and are in need of ports<br />
to get venous access. This means a surgical procedure<br />
early in life with its risks. Also, surgical procedures<br />
have been suggested to be a risk factor for<br />
inhibitor deve<strong>lo</strong>pment. 32 Novel products with <strong>lo</strong>nger<br />
b<strong>io</strong><strong>lo</strong>gical half-life have a potential to increase convenience<br />
and reduce the need of port implantat<strong>io</strong>ns.<br />
Such products are now in clinical trials.<br />
Figure 1. Computer modeling of pharmacokinetic dosing where PK parameters for the patient are known. Dosing schedules<br />
were modeled to have similar trough levels. Shortening the intervals will dramatically reduce factor consumpt<strong>io</strong>n,<br />
but also peak levels. The clinical impact of the different schedules remains to be evaluated. The standard dose for this<br />
patient is 2000 units 3 times per week (Adopted from ref. 21 ).<br />
Hemato<strong>lo</strong>gy Educat<strong>io</strong>n: the educat<strong>io</strong>n programme for the annual congress of the <strong>European</strong> Hemato<strong>lo</strong>gy Associat<strong>io</strong>n | 2011; 5(1) | 85 |