H e m a t o lo g y E d u c a t io n - European Hematology Association
H e m a t o lo g y E d u c a t io n - European Hematology Association
H e m a t o lo g y E d u c a t io n - European Hematology Association
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
was thought that they are linked to a pure erythrocytosis<br />
phenotype. However, exon 12 JAK2 mutat<strong>io</strong>n<br />
patients deve<strong>lo</strong>p thrombosis complicat<strong>io</strong>ns, secondary<br />
mye<strong>lo</strong>fibrosis, and leukemia similarly to those harboring<br />
JAK2 V617F. 30<br />
Thrombopoietin receptor mutat<strong>io</strong>ns<br />
Approximately 8–10% of ET and PMF patients that<br />
do not harbor JAK2 V617F carry mutat<strong>io</strong>ns in the TpoR.<br />
Fascinatingly, most mutat<strong>io</strong>ns seem to concern<br />
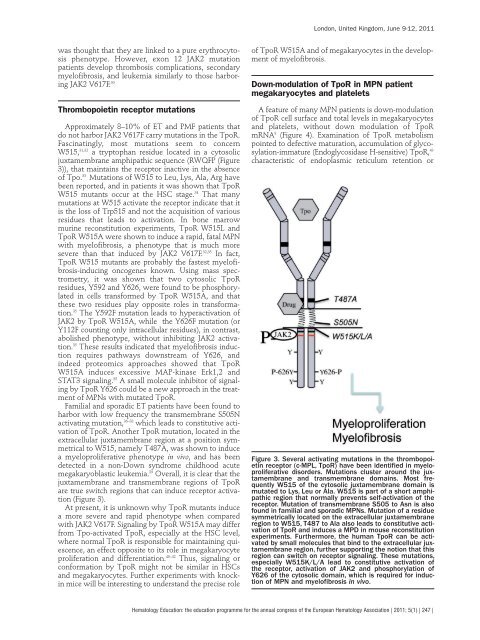
W515, 31,32 a tryptophan residue <strong>lo</strong>cated in a cytosolic<br />
juxtamembrane amphipathic sequence (RWQFP (Figure<br />
3)), that maintains the receptor inactive in the absence<br />
of Tpo. 33 Mutat<strong>io</strong>ns of W515 to Leu, Lys, Ala, Arg have<br />
been reported, and in patients it was shown that TpoR<br />
W515 mutants occur at the HSC stage. 34 That many<br />
mutat<strong>io</strong>ns at W515 activate the receptor indicate that it<br />
is the <strong>lo</strong>ss of Trp515 and not the acquisit<strong>io</strong>n of var<strong>io</strong>us<br />
residues that leads to activat<strong>io</strong>n. In bone marrow<br />
murine reconstitut<strong>io</strong>n experiments, TpoR W515L and<br />
TpoR W515A were shown to induce a rapid, fatal MPN<br />
with mye<strong>lo</strong>fibrosis, a phenotype that is much more<br />
severe than that induced by JAK2 V617F. 32,35 In fact,<br />
TpoR W515 mutants are probably the fastest mye<strong>lo</strong>fibrosis-inducing<br />
oncogenes known. Using mass spectrometry,<br />
it was shown that two cytosolic TpoR<br />
residues, Y592 and Y626, were found to be phosphorylated<br />
in cells transformed by TpoR W515A, and that<br />
these two residues play opposite roles in transformat<strong>io</strong>n.<br />
35 The Y592F mutat<strong>io</strong>n leads to hyperactivat<strong>io</strong>n of<br />
JAK2 by TpoR W515A, while the Y626F mutat<strong>io</strong>n (or<br />
Y112F counting only intracellular residues), in contrast,<br />
abolished phenotype, without inhibiting JAK2 activat<strong>io</strong>n.<br />
35 These results indicated that mye<strong>lo</strong>fibrosis induct<strong>io</strong>n<br />
requires pathways downstream of Y626, and<br />
indeed proteomics approaches showed that TpoR<br />
W515A induces excessive MAP-kinase Erk1,2 and<br />
STAT3 signaling. 35 A small molecule inhibitor of signaling<br />
by TpoR Y626 could be a new approach in the treatment<br />
of MPNs with mutated TpoR.<br />
Familial and sporadic ET patients have been found to<br />
harbor with <strong>lo</strong>w frequency the transmembrane S505N<br />
activating mutat<strong>io</strong>n, 36–38 which leads to constitutive activat<strong>io</strong>n<br />
of TpoR. Another TpoR mutat<strong>io</strong>n, <strong>lo</strong>cated in the<br />
extracellular juxtamembrane reg<strong>io</strong>n at a posit<strong>io</strong>n symmetrical<br />
to W515, namely T487A, was shown to induce<br />
a mye<strong>lo</strong>proliferative phenotype in vivo, and has been<br />
detected in a non-Down syndrome childhood acute<br />
megakaryoblastic leukemia. 39 Overall, it is clear that the<br />
juxtamembrane and transmembrane reg<strong>io</strong>ns of TpoR<br />
are true switch reg<strong>io</strong>ns that can induce receptor activat<strong>io</strong>n<br />
(Figure 3).<br />
At present, it is unknown why TpoR mutants induce<br />
a more severe and rapid phenotype when compared<br />
with JAK2 V617F. Signaling by TpoR W515A may differ<br />
from Tpo-activated TpoR, especially at the HSC level,<br />
where normal TpoR is responsible for maintaining quiescence,<br />
an effect opposite to its role in megakaryocyte<br />
proliferat<strong>io</strong>n and differentiat<strong>io</strong>n. 40–42 Thus, signaling or<br />
conformat<strong>io</strong>n by TpoR might not be similar in HSCs<br />
and megakaryocytes. Further experiments with knockin<br />
mice will be interesting to understand the precise role<br />
London, United Kingdom, June 9-12, 2011<br />
of TpoR W515A and of megakaryocytes in the deve<strong>lo</strong>pment<br />
of mye<strong>lo</strong>fibrosis.<br />
Down-modulat<strong>io</strong>n of TpoR in MPN patient<br />
megakaryocytes and platelets<br />
A feature of many MPN patients is down-modulat<strong>io</strong>n<br />
of TpoR cell surface and total levels in megakaryocytes<br />
and platelets, without down modulat<strong>io</strong>n of TpoR<br />
mRNA 8 (Figure 4). Examinat<strong>io</strong>n of TpoR metabolism<br />
pointed to defective maturat<strong>io</strong>n, accumulat<strong>io</strong>n of glycosylat<strong>io</strong>n-immature<br />
(Endoglycosidase H-sensitive) TpoR, 43<br />
characteristic of endoplasmic reticulum retent<strong>io</strong>n or<br />
Figure 3. Several activating mutat<strong>io</strong>ns in the thrombopoietin<br />
receptor (c-MPL, TpoR) have been identified in mye<strong>lo</strong>proliferative<br />
disorders. Mutat<strong>io</strong>ns cluster around the juxtamembrane<br />
and transmembrane domains. Most frequently<br />
W515 of the cytosolic juxtamembrane domain is<br />
mutated to Lys, Leu or Ala. W515 is part of a short amphipathic<br />
reg<strong>io</strong>n that normally prevents self-activat<strong>io</strong>n of the<br />
receptor. Mutat<strong>io</strong>n of transmembrane S505 to Asn is also<br />
found in familial and sporadic MPNs. Mutat<strong>io</strong>n of a residue<br />
symmetrically <strong>lo</strong>cated on the extracellular juxtamembrane<br />
reg<strong>io</strong>n to W515, T487 to Ala also leads to constitutive activat<strong>io</strong>n<br />
of TpoR and induces a MPD in mouse reconstitut<strong>io</strong>n<br />
experiments. Furthermore, the human TpoR can be activated<br />
by small molecules that bind to the extracellular juxtamembrane<br />
reg<strong>io</strong>n, further supporting the not<strong>io</strong>n that this<br />
reg<strong>io</strong>n can switch on receptor signaling. These mutat<strong>io</strong>ns,<br />
especially W515K/L/A lead to constitutive activat<strong>io</strong>n of<br />
the receptor, activat<strong>io</strong>n of JAK2 and phosphorylat<strong>io</strong>n of<br />
Y626 of the cytosolic domain, which is required for induct<strong>io</strong>n<br />
of MPN and mye<strong>lo</strong>fibrosis in vivo.<br />
Hemato<strong>lo</strong>gy Educat<strong>io</strong>n: the educat<strong>io</strong>n programme for the annual congress of the <strong>European</strong> Hemato<strong>lo</strong>gy Associat<strong>io</strong>n | 2011; 5(1) | 247 |