et al.
et al.
et al.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
RESEARCH ARTICLES<br />
784<br />
Another possibility is that NWA 7034 origin<strong>al</strong>ly<br />
had oxygen isotope v<strong>al</strong>ues similar to or the<br />
same as SNC, but a com<strong>et</strong>ary component with<br />
higher d 18 O, d 17 O, and D 17 O was mixed with it<br />
through impact processes on Mars, thus producing<br />
a D 17 O excess relative to SNC. Until we find<br />
clear evidence of such an exotic component in<br />
NWA 7034, this scenario seems less likely than the<br />
other two.<br />
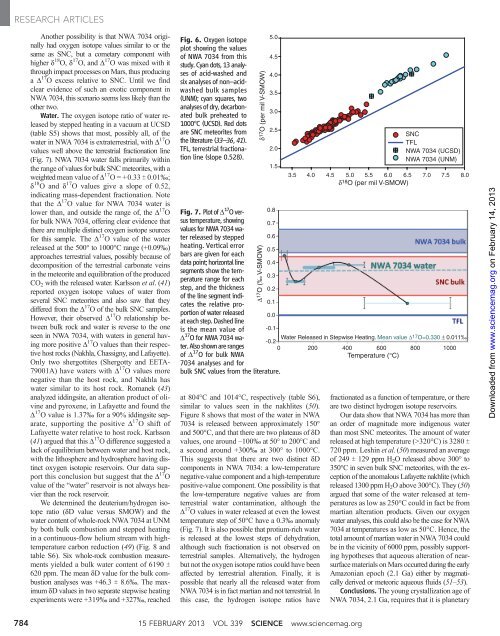
Water. The oxygen isotope ratio of water released<br />
by stepped heating in a vacuum at UCSD<br />
(table S5) shows that most, possibly <strong>al</strong>l, of the<br />
water in NWA 7034 is extraterrestri<strong>al</strong>, with D 17 O<br />
v<strong>al</strong>ues well above the terrestri<strong>al</strong> fractionation line<br />
(Fig. 7). NWA 7034 water f<strong>al</strong>ls primarily within<br />
the range of v<strong>al</strong>ues for bulk SNC m<strong>et</strong>eorites, with a<br />
weighted mean v<strong>al</strong>ue of D 17 O = +0.33 T 0.01‰;<br />
d 18 O and d 17 O v<strong>al</strong>ues give a slope of 0.52,<br />
indicating mass-dependent fractionation. Note<br />
that the D 17 O v<strong>al</strong>ue for NWA 7034 water is<br />
lower than, and outside the range of, the D 17 O<br />
for bulk NWA 7034, offering clear evidence that<br />
there are multiple distinct oxygen isotope sources<br />
for this sample. The D 17 O v<strong>al</strong>ue of the water<br />
released at the 500° to 1000°C range (+0.09‰)<br />
approaches terrestri<strong>al</strong> v<strong>al</strong>ues, possibly because of<br />
decomposition of the terrestri<strong>al</strong> carbonate veins<br />
in the m<strong>et</strong>eorite and equilibration of the produced<br />
CO 2 with the released water. Karlsson <strong>et</strong> <strong>al</strong>.(41)<br />
reported oxygen isotope v<strong>al</strong>ues of water from<br />
sever<strong>al</strong> SNC m<strong>et</strong>eorites and <strong>al</strong>so saw that they<br />
differed from the D 17 O of the bulk SNC samples.<br />
However, their observed D 17 O relationship b<strong>et</strong>ween<br />
bulk rock and water is reverse to the one<br />
seen in NWA 7034, with waters in gener<strong>al</strong> having<br />
more positive D 17 O v<strong>al</strong>ues than their respective<br />
host rocks (Nakhla, Chassigny, and Lafay<strong>et</strong>te).<br />
Only two shergottites (Shergotty and EETA-<br />
79001A) have waters with D 17 O v<strong>al</strong>ues more<br />
negative than the host rock, and Nakhla has<br />
water similar to its host rock. Romanek (43)<br />
an<strong>al</strong>yzed iddingsite, an <strong>al</strong>teration product of olivine<br />
and pyroxene, in Lafay<strong>et</strong>te and found the<br />
D 17 O v<strong>al</strong>ue is 1.37‰ for a 90% iddingsite separate,<br />
supporting the positive D 17 O shift of<br />
Lafay<strong>et</strong>te water relative to host rock. Karlsson<br />
(41) argued that this D 17 O difference suggested a<br />
lack of equilibrium b<strong>et</strong>ween water and host rock,<br />
with the lithosphere and hydrosphere having distinct<br />
oxygen isotopic reservoirs. Our data support<br />
this conclusion but suggest that the D 17 O<br />
v<strong>al</strong>ue of the “water” reservoir is not <strong>al</strong>ways heavier<br />
than the rock reservoir.<br />
We d<strong>et</strong>ermined the deuterium/hydrogen isotope<br />
ratio (dD v<strong>al</strong>ue versus SMOW) and the<br />
water content of whole-rock NWA 7034 at UNM<br />
by both bulk combustion and stepped heating<br />
in a continuous-flow helium stream with hightemperature<br />
carbon reduction (49) (Fig. 8 and<br />
table S6). Six whole-rock combustion measurements<br />
yielded a bulk water content of 6190 T<br />
620 ppm. The mean dD v<strong>al</strong>ue for the bulk combustion<br />
an<strong>al</strong>yses was +46.3 T 8.6‰. The maximum<br />
dD v<strong>al</strong>ues in two separate stepwise heating<br />
experiments were +319‰ and +327‰, reached<br />
Fig. 6. Oxygen isotope<br />
plot showing the v<strong>al</strong>ues<br />
of NWA 7034 from this<br />
study. Cyan dots, 13 an<strong>al</strong>yses<br />
of acid-washed and<br />
six an<strong>al</strong>yses of non–acidwashed<br />
bulk samples<br />
(UNM); cyan squares, two<br />
an<strong>al</strong>yses of dry, decarbonated<br />
bulk preheated to<br />
1000°C (UCSD). Red dots<br />
are SNC m<strong>et</strong>eorites from<br />
the literature (33–36, 41).<br />
TFL, terrestri<strong>al</strong> fractionationline(slope0.528).<br />
Fig. 7. Plot of D 17 Oversus<br />
temperature, showing<br />
v<strong>al</strong>ues for NWA 7034 water<br />
released by stepped<br />
heating. Vertic<strong>al</strong> error<br />
bars are given for each<br />
data point; horizont<strong>al</strong> line<br />
segments show the temperature<br />
range for each<br />
step, and the thickness<br />
of the line segment indicates<br />
the relative proportion<br />
of water released<br />
at each step. Dashed line<br />
is the mean v<strong>al</strong>ue of<br />
D 17 OforNWA7034water.<br />
Also shown are ranges<br />
of D 17 OforbulkNWA<br />
7034 an<strong>al</strong>yses and for<br />
δ 17 O (per mil V-SMOW)<br />
5.0<br />
4.5<br />
4.0<br />
3.5<br />
3.0<br />
2.5<br />
2.0<br />
1.5<br />
-0.2<br />
0<br />
bulk SNC v<strong>al</strong>ues from the literature.<br />
at 804°C and 1014°C, respectively (table S6),<br />
similar to v<strong>al</strong>ues seen in the nakhlites (50).<br />
Figure 8 shows that most of the water in NWA<br />
7034 is released b<strong>et</strong>ween approximately 150°<br />
and 500°C, and that there are two plateaus of dD<br />
v<strong>al</strong>ues, one around –100‰ at 50° to 200°C and<br />
a second around +300‰ at 300° to 1000°C.<br />
This suggests that there are two distinct dD<br />
components in NWA 7034: a low-temperature<br />
negative-v<strong>al</strong>ue component and a high-temperature<br />
positive-v<strong>al</strong>ue component. One possibility is that<br />
the low-temperature negative v<strong>al</strong>ues are from<br />
terrestri<strong>al</strong> water contamination, <strong>al</strong>though the<br />
D 17 O v<strong>al</strong>ues in water released at even the lowest<br />
temperature step of 50°C have a 0.3‰ anom<strong>al</strong>y<br />
(Fig. 7). It is <strong>al</strong>so possible that protium-rich water<br />
is released at the lowest steps of dehydration,<br />
<strong>al</strong>though such fractionation is not observed on<br />
terrestri<strong>al</strong> samples. Alternatively, the hydrogen<br />
but not the oxygen isotope ratios could have been<br />
affected by terrestri<strong>al</strong> <strong>al</strong>teration. Fin<strong>al</strong>ly, it is<br />
possible that nearly <strong>al</strong>l the released water from<br />
NWA 7034 is in fact martian and not terrestri<strong>al</strong>. In<br />
this case, the hydrogen isotope ratios have<br />
15 FEBRUARY 2013 VOL 339 SCIENCE www.sciencemag.org<br />
∆ 17 O (‰ V-SMOW)<br />
0.8<br />
0.7<br />
0.6<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
0.0<br />
-0.1<br />
SNC<br />
TFL<br />
NWA 7034 (UCSD)<br />
NWA 7034 (UNM)<br />
3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 8.0<br />
δ18O (per mil V-SMOW)<br />
Water Released in Stepwise Heating. Mean v<strong>al</strong>ue ∆17O=0.330 ± 0.011‰<br />
200 400 600 800 1000<br />
Temperature (°C)<br />
fractionated as a function of temperature, or there<br />
are two distinct hydrogen isotope reservoirs.<br />
Our data show that NWA 7034 has more than<br />
an order of magnitude more indigenous water<br />
than most SNC m<strong>et</strong>eorites. The amount of water<br />
released at high temperature (>320°C) is 3280 T<br />
720 ppm. Leshin <strong>et</strong> <strong>al</strong>.(50) measured an average<br />
of 249 T 129 ppm H2O released above 300° to<br />
350°C in seven bulk SNC m<strong>et</strong>eorites, with the exception<br />
of the anom<strong>al</strong>ous Lafay<strong>et</strong>te nakhlite (which<br />
released 1300 ppm H2O above 300°C). They (50)<br />
argued that some of the water released at temperatures<br />
as low as 250°C could in fact be from<br />
martian <strong>al</strong>teration products. Given our oxygen<br />
water an<strong>al</strong>yses, this could <strong>al</strong>so be the case for NWA<br />
7034 at temperatures as low as 50°C. Hence, the<br />
tot<strong>al</strong> amount of martian water in NWA 7034 could<br />
be in the vicinity of 6000 ppm, possibly supporting<br />
hypotheses that aqueous <strong>al</strong>teration of nearsurface<br />
materi<strong>al</strong>s on Mars occurred during the early<br />
Amazonian epoch (2.1 Ga) either by magmatic<strong>al</strong>ly<br />
derived or m<strong>et</strong>eoric aqueous fluids (51–53).<br />
Conclusions. The young cryst<strong>al</strong>lization age of<br />
NWA 7034, 2.1 Ga, requires that it is plan<strong>et</strong>ary<br />
on February 14, 2013<br />
www.sciencemag.org<br />
Downloaded from