Figure I Generalized map of the Wilbur Mining ... - University of Utah
Figure I Generalized map of the Wilbur Mining ... - University of Utah
Figure I Generalized map of the Wilbur Mining ... - University of Utah
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Thompson<br />
T OC<br />
pH<br />
SIO2<br />
Al<br />
Fe<br />
Mn<br />
Ca<br />
Mg<br />
Sr<br />
Ba<br />
Na<br />
K<br />
Ll<br />
NHi|<br />
HCO 3<br />
CO3<br />
so^<br />
Cl<br />
F<br />
Br<br />
I<br />
B<br />
H2S<br />
Na-K-•Ca<br />
Mg corr<br />
SIO2<br />
Na-K-Ca<br />
Adlabatic<br />
Conductive<br />
^Analyses,in mg/L<br />
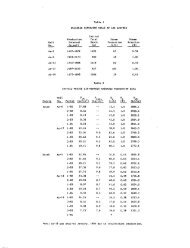
Table 1. Averaged concentrations for spring and well water<br />
in <strong>the</strong> <strong>Wilbur</strong> mining district'<br />
<strong>Wilbur</strong><br />
Hot Spring<br />
52<br />
7.5<br />
176<br />
1.8<br />
0.17<br />
O.Of<br />
2.5<br />
15<br />
3.6<br />
3.1<br />
8700<br />
^08<br />
11.6<br />
29'*<br />
6900<br />
—<br />
356(H)<br />
9980<br />
2.1<br />
19<br />
12<br />
233<br />
165<br />
236<br />
8it<br />
218<br />
_—_<br />
Jones'<br />
Fountain <strong>of</strong><br />
Life<br />
60<br />
7.7<br />
89<br />
0.35<br />
0.05<br />
2.6<br />
31<br />
1.6<br />
3.0<br />
9880<br />
it 32<br />
10.7<br />
120<br />
57IO<br />
—<br />
71<br />
11,700<br />
3.5<br />
31<br />
23<br />
2H0<br />
232<br />
118<br />
223<br />
to <strong>Wilbur</strong> Hot Springs (see Table 1) <strong>Wilbur</strong> ffl<br />
geo<strong>the</strong>nnal well contains (1) a higher chloride<br />
content (11,100 vs. 10,000 mg/L), (2) a lower<br />
sulfate content (260 vs. 36O mg/L) and (3) a much<br />
lower magnesium content (2 vs. 15 mg/L).<br />
The magnesium corrected Na-K-Ca geo<strong>the</strong>rmometer<br />
(Fournier and Potter, 1978) indicates temperatures<br />
ranging from 10 to 160°C. However, because <strong>the</strong><br />
country rock around <strong>Wilbur</strong> Hot Springs is<br />
principally serpentinite, <strong>the</strong> high magnesium in<br />
<strong>Wilbur</strong> Hot Spring is probably due to serpentine<br />
dissolution. The uncorrected Na-K-Ca (Fournier<br />
and Truesdell, 1973) temperatures, which range<br />
from 220 to 218°C, may be more reasonable.<br />
Water from <strong>Wilbur</strong> ff 1 geo<strong>the</strong>rmal well has a<br />
Na-K-Ca temperature <strong>of</strong> 2IIOC (Table 1) and very<br />
little magnesium; a correction <strong>of</strong> only 1°C is<br />
calculated.<br />
Due to possible silica addition from<br />
serpentine dissolution, severe difficulties are<br />
encountered when using dissolved silica<br />
Blanck's<br />
Spring<br />
12<br />
7.8<br />
121<br />
0.19<br />
0.05<br />
3.5<br />
69<br />
1.1<br />
1.5<br />
7220<br />
360<br />
6.9<br />
125<br />
6390<br />
—<br />
180<br />
8050<br />
2.3<br />
21<br />
18<br />
150<br />
730<br />
232<br />
16<br />
198<br />
——<br />
Elgin Mine<br />
Springs<br />
61<br />
198<br />
0.17<br />
1.0<br />
1.8<br />
28<br />
3.7<br />
9330<br />
510<br />
11<br />
213<br />
7270<br />
—<br />
86<br />
11,550<br />
3.2<br />
30<br />
25<br />
210<br />
170<br />
211<br />
118<br />
208<br />
— •'—<br />
<strong>Wilbur</strong> fi<br />
Geo<strong>the</strong>rmal<br />
well<br />
110<br />
8.8<br />
133<br />
— 1<br />
2<br />
10,000<br />
110<br />
—<br />
275<br />
5170<br />
1170<br />
263<br />
11,100<br />
16<br />
—<br />
• —<br />
118<br />
211<br />
213<br />
201<br />
Meteoric<br />
water<br />
19.5<br />
6.8<br />
21<br />
— 3.1<br />
182<br />
—<br />
—<br />
132<br />
1.1<br />
0.16<br />
—<br />
1020<br />
0<br />
185<br />
83<br />
.37<br />
—<br />
—<br />
conoencration in estimating <strong>the</strong>rmal reservoir<br />
temperatures. The difficulties include <strong>the</strong><br />
following: (1) <strong>the</strong> silica may have already<br />
polymerized or precipitated so that direct<br />
application <strong>of</strong> <strong>the</strong> silica geo<strong>the</strong>rmometer (Fournier<br />
and Rowe, 1966) will indicate a low reservoir<br />
temperature; (2) <strong>the</strong> <strong>the</strong>rmal water is probably<br />
mixed with dilute meteoric water giving rise to<br />
<strong>the</strong> observed spring water compositions and<br />
temperatures; and (3) <strong>the</strong>.diluting water or <strong>the</strong><br />
warm mixed water may contain some silica<br />
originating from low-temperature serpentine<br />
dissolution. For comparison, <strong>the</strong> conductive and<br />
adiabatic (with assumed subsurface steam loss at<br />
lOQOC) silica-mixing-model temperatures<br />
(Truesdell and Fournier, 1977) <strong>of</strong> <strong>the</strong> warm springs<br />
are shown in Table 1. Despite all <strong>of</strong> <strong>the</strong> possible<br />
problems using silica concentrations in springs<br />
from this area, <strong>the</strong> adiabatic mixed-water<br />
temperatures are in moderate to good agreement<br />
with <strong>the</strong> Na-K-Ca temperatures. Fournier (1979)'<br />
indicated that silica reequilibratlon is more<br />
likely to occur than Na-K-Ca reequilibratlon. The<br />
—