Figure I Generalized map of the Wilbur Mining ... - University of Utah
Figure I Generalized map of the Wilbur Mining ... - University of Utah
Figure I Generalized map of the Wilbur Mining ... - University of Utah
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
This method <strong>of</strong> calculation assumes <strong>the</strong> following:<br />
1. Depth is a vertical distance from ground surface.<br />
2. Correction for mean annual temperature is independent<br />
<strong>of</strong> discharge rate.<br />
3. Water is not mixing between aquifers.<br />
4. Temperature equilibrium is maintained in well.<br />
5. Mean annual temperature influences water temperature<br />
at surface and influences critical<br />
depth as defined above.<br />
CHEMICAL COMPOSITION<br />
The results <strong>of</strong> standard mineral analyses were<br />
plotted on multiple tri-linear diagr'ams after<br />
Piper (1944). Ground waters were classified as<br />
ei<strong>the</strong>r sodium chloride-calcium sulfate type, calcium<br />
sulfate type, or calcium-magnesium bicarbonate<br />
type. The bicarbonate waters were generally<br />
found in wells and springs near recharge areas,<br />
and <strong>the</strong> calcium sulfate waters were found with<br />
ei<strong>the</strong>r increasing distance from recharge areas<br />
or in ground waters which were known to be mixing<br />
between aquifers. The sodium chloride-calcium<br />
sulfate type water was found only in Madison<br />
ground watiers in <strong>the</strong> Edgemont geo<strong>the</strong>nnal area.<br />
The standard mineral composition <strong>of</strong> <strong>the</strong>se ground<br />
waters was found to be significantly different<br />
from that <strong>of</strong> all o<strong>the</strong>r sampled ground waters.<br />
Of all <strong>the</strong> trace elements analyzed, only lithium<br />
and cobalt have higher concentrations in Edgemont<br />
area waters than in waters at all o<strong>the</strong>r sites.<br />
Strontium concentrations in Edgemont area waters<br />
ranged from 1,8 to 3,4 mg/l. Although cesium and<br />
rubidium were detected in Edgemont ground waters,<br />
it was not determined whe<strong>the</strong>r this is significant<br />
as <strong>the</strong>re is no data from o<strong>the</strong>r sampled sites.<br />
Aluminum, copper, lead, manganese, silver, and<br />
zinc were not detected in Edgemont waters, and<br />
additional analyses supplied by <strong>the</strong> U.S. Geological<br />
Survey indicated that only trace amounts <strong>of</strong><br />
<strong>the</strong>se elements, if any at all, occur in ground<br />
waters at o<strong>the</strong>r sites in <strong>the</strong> study area.<br />
GEOTHERMOMETRY<br />
Temperatures were calculated using <strong>the</strong> quartz and<br />
chalcedony geo<strong>the</strong>rmometers defined by Truesdell<br />
(1975) and <strong>the</strong> Na-K-Ca geo<strong>the</strong>rmometer defined by<br />
Fournier and Truesdell (1973). Water temperatures<br />
calculated using <strong>the</strong> quartz geo<strong>the</strong>rmometer ranged<br />
from 9.70c to 46.IOC above observed temperatures,<br />
whereas temperatures calculated using <strong>the</strong> chalcedony<br />
geo<strong>the</strong>rmometer ranged from 24.50C below to<br />
14.0OC above observed temperatures. Water temperature<br />
calculated using <strong>the</strong> Na-K-Ca geo<strong>the</strong>rmometer<br />
ranged from 41.30C below to 41.50C above observed<br />
temperatures.<br />
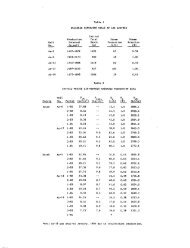
Table 3 lists <strong>the</strong> results <strong>of</strong> selected geo<strong>the</strong>rmometer<br />
calculations. The chalcedony geo<strong>the</strong>rmometer<br />
yielded <strong>the</strong> best correlation between observed temperatures<br />
and calculated temperatures. This is in<br />
agreerent with Fournier (1973) who found that<br />
chalcedony, ra<strong>the</strong>r than quartz, controls <strong>the</strong> dissolved<br />
silica content <strong>of</strong> waters below lOOOC.<br />
167<br />
Knirsch<br />
Most work with geo<strong>the</strong>rmometers has been done for<br />
high-temperature geo<strong>the</strong>rmal systems. The highest<br />
observed temperature in this study was 560C. No<br />
correlations exist between <strong>the</strong> results <strong>of</strong> geo<strong>the</strong>rmometer<br />
calculations and temperature-depth gradients.<br />
ACKNOWLEDGEMENTS<br />
We wish to thank <strong>the</strong> U.S. Department <strong>of</strong> Energy and<br />
<strong>the</strong> U.S. Geological Survey for <strong>the</strong>ir assistance<br />
with this study.<br />
REFERENCES<br />
Fournier, R. 0., 1973, Silica in <strong>the</strong>rmal waters:<br />
Laboratory and Field Investigations, 2£ Proceedings<br />
<strong>of</strong> <strong>the</strong> International Symposium on Hydrogeochemistry<br />
and Biogeochemistry, Japan, 1970, v.<br />
I, Hydrogeochemistry: Washington, DC, The Clark<br />
Co., p. 122-139.<br />
Fournier, R, 0., and Truesdell, A. H., 1973, An<br />
empirical Na-K-Ca geo<strong>the</strong>rmometer for natural<br />
waters: Geochim. et Cosmochim. Acta, v. 37,<br />
n. 5, p. 1255-1276.<br />
Gries, J. P., 1977, Geo<strong>the</strong>nnal applications on <strong>the</strong><br />
Madison (Pahasapa) aquifer system in South Dakota:<br />
Final Report, Contract No. EY-76-S-07-1625,<br />
South Dakota School <strong>of</strong> Mines and Technology,<br />
100 pp.<br />
Iszler, J., Carda, D,, Skillman, D., Dunham, G.,<br />
and Reuter, W., 1979, Western South Dakota usage<br />
<strong>of</strong> geo<strong>the</strong>rmal energy from <strong>the</strong> Madison Formation<br />
--final report, ET-78-S-07-1707: Department <strong>of</strong><br />
Energy, Division <strong>of</strong> Geo<strong>the</strong>rmal Energy, Washington,<br />
D.C., 153 pp.<br />
Piper, A. M., 1944, A graphic procedure in <strong>the</strong><br />
geochemical interpretation <strong>of</strong> water analyses:<br />
Trans., Am. Geophys. Union, 25th annual meeting,<br />
p. 914-923.<br />
Schoon, R. A., and McGregor, D. J., 1974, Geo<strong>the</strong>rmal<br />
potentials in South Dakota: S.O. Geol.<br />
Survey Rept. Inv. 110, 76 pp.<br />
Truesdell, A. H., 1975, Summary <strong>of</strong> Section III:<br />
Geochemical techniques in exploration Ui Proceedings,<br />
United Nations Symposium on <strong>the</strong> Development<br />
and Use <strong>of</strong> Geo<strong>the</strong>rmal Resources, 2nd,<br />
San Francisco: Washington, D.C, U.S. Govt.<br />
Printing Office, p. LIII-LXXIX.