Introductory Physics Volume Two
Introductory Physics Volume Two
Introductory Physics Volume Two
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
-<br />
-<br />
-<br />
-<br />
-<br />
1.2 Coulomb’s Law 7<br />
How can we understand this apparently new type of charge, a<br />
charge that is attracted to both positive and negative charges? The<br />
place to start is consider the aluminum of which the can is composed.<br />
Each aluminum atom is composed of an equal number of protons and<br />
electrons, so the net charge of the can is zero. But there is a difference<br />
between the protons and electrons. The aluminum atoms are locked<br />
together in a crystal lattice, with the protons locked together with the<br />
neutrons in the nucleus of the atoms. The protons cannot move. On the<br />
other hand some of the electrons are shared between all of the atoms,<br />
and move around freely. This is what makes aluminum a conductor, it<br />
has electrons that move freely through the bulk of the material.<br />
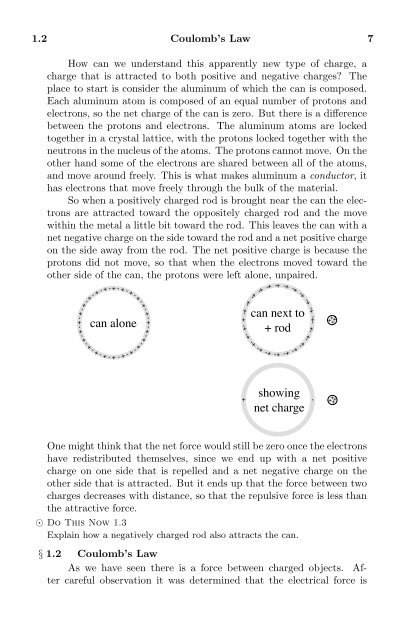
So when a positively charged rod is brought near the can the electrons<br />
are attracted toward the oppositely charged rod and the move<br />
within the metal a little bit toward the rod. This leaves the can with a<br />
net negative charge on the side toward the rod and a net positive charge<br />
on the side away from the rod. The net positive charge is because the<br />
protons did not move, so that when the electrons moved toward the<br />
other side of the can, the protons were left alone, unpaired.<br />
+<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
can alone<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
+<br />
-<br />
+<br />
-<br />
+<br />
+<br />
+<br />
-<br />
+<br />
-<br />
-<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
+<br />
-<br />
+<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
-<br />
can next to<br />
+ rod<br />
-<br />
-<br />
-<br />
-<br />
-<br />
+<br />
+<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
-<br />
+<br />
+<br />
+<br />
+<br />
+<br />
-<br />
-<br />
+ + + +<br />
-<br />
-<br />
-<br />
-<br />
+<br />
showing<br />
net charge<br />
+ + + +<br />
One might think that the net force would still be zero once the electrons<br />
have redistributed themselves, since we end up with a net positive<br />
charge on one side that is repelled and a net negative charge on the<br />
other side that is attracted. But it ends up that the force between two<br />
charges decreases with distance, so that the repulsive force is less than<br />
the attractive force.<br />
⊙ Do This Now 1.3<br />
Explain how a negatively charged rod also attracts the can.<br />
§ 1.2 Coulomb’s Law<br />
As we have seen there is a force between charged objects. After<br />
careful observation it was determined that the electrical force is