Innovation in European healthcare â what can Sweden learn? - LIF
Innovation in European healthcare â what can Sweden learn? - LIF
Innovation in European healthcare â what can Sweden learn? - LIF
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
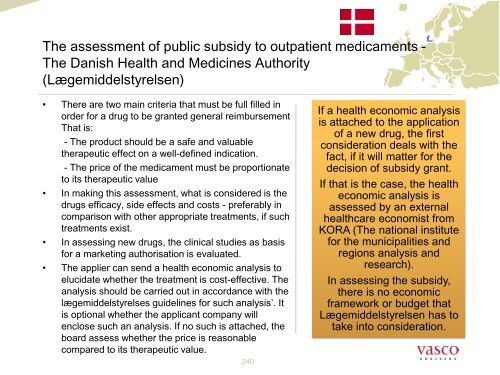
The assessment of public subsidy to outpatient medicaments -<br />
The Danish Health and Medic<strong>in</strong>es Authority<br />
(Lægemiddelstyrelsen)<br />
• There are two ma<strong>in</strong> criteria that must be full filled <strong>in</strong><br />
order for a drug to be granted general reimbursement<br />
That is:<br />
- The product should be a safe and valuable<br />
therapeutic effect on a well-def<strong>in</strong>ed <strong>in</strong>dication.<br />
- The price of the medicament must be proportionate<br />
to its therapeutic value<br />
• In mak<strong>in</strong>g this assessment, <strong>what</strong> is considered is the<br />
drugs efficacy, side effects and costs - preferably <strong>in</strong><br />
comparison with other appropriate treatments, if such<br />
treatments exist.<br />
• In assess<strong>in</strong>g new drugs, the cl<strong>in</strong>ical studies as basis<br />
for a market<strong>in</strong>g authorisation is evaluated.<br />
• The applier <strong>can</strong> send a health economic analysis to<br />
elucidate whether the treatment is cost-effective. The<br />
analysis should be carried out <strong>in</strong> accordance with the<br />
lægemiddelstyrelses guidel<strong>in</strong>es for such analysis’. It<br />
is optional whether the appli<strong>can</strong>t company will<br />
enclose such an analysis. If no such is attached, the<br />
board assess whether the price is reasonable<br />
compared to its therapeutic value.<br />
240<br />
If a health economic analysis<br />
is attached to the application<br />
of a new drug, the first<br />
consideration deals with the<br />
fact, if it will matter for the<br />
decision of subsidy grant.<br />
If that is the case, the health<br />
economic analysis is<br />
assessed by an external<br />
<strong>healthcare</strong> economist from<br />
KORA (The national <strong>in</strong>stitute<br />
for the municipalities and<br />
regions analysis and<br />
research).<br />
In assess<strong>in</strong>g the subsidy,<br />
there is no economic<br />
framework or budget that<br />
Lægemiddelstyrelsen has to<br />
take <strong>in</strong>to consideration.