V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
V.D.2 Interfacial Behavior <strong>of</strong> Electrolytes (LBNL)<br />
Kerr – LBNL<br />
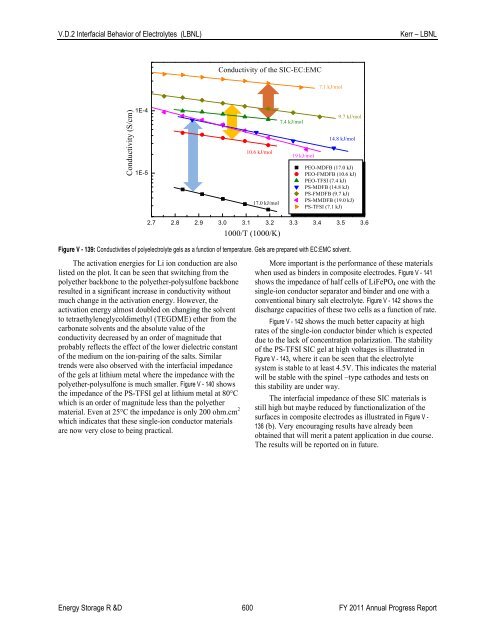
Conductivity <strong>of</strong> the SIC-EC:EMC<br />
7.1 kJ/mol<br />
Conductivity (S/cm)<br />
1E-4<br />
1E-5<br />
10.6 kJ/mol<br />
17.0 kJ/mol<br />
7.4 kJ/mol<br />
19 kJ/mol<br />
9.7 kJ/mol<br />
14.8 kJ/mol<br />
PEO-MDFB (17.0 kJ)<br />
PEO-FMDFB (10.6 kJ)<br />
PEO-TFSI (7.4 kJ)<br />
PS-MDFB (14.8 kJ)<br />
PS-FMDFB (9.7 kJ)<br />
PS-MMDFB (19.0 kJ)<br />
PS-TFSI (7.1 kJ)<br />
2.7 2.8 2.9 3.0 3.1 3.2 3.3 3.4 3.5 3.6<br />
1000/T (1000/K)<br />
Figure V - 139: Conductivities <strong>of</strong> polyelectrolyte gels as a function <strong>of</strong> temperature. Gels are prepared with EC:EMC solvent.<br />
The activation energies for Li ion conduction are also<br />
listed on the plot. It can be seen that switching from the<br />
polyether backbone to the polyether-polysulfone backbone<br />
resulted in a significant increase in conductivity without<br />
much change in the activation energy. However, the<br />
activation energy almost doubled on changing the solvent<br />
to tetraethyleneglycoldimethyl (TEGDME) ether from the<br />
carbonate solvents and the absolute value <strong>of</strong> the<br />
conductivity decreased by an order <strong>of</strong> magnitude that<br />
probably reflects the effect <strong>of</strong> the lower dielectric constant<br />
<strong>of</strong> the medium on the ion-pairing <strong>of</strong> the salts. Similar<br />
trends were also observed with the interfacial impedance<br />
<strong>of</strong> the gels at lithium metal where the impedance with the<br />
polyether-polysulfone is much smaller. Figure V - 140 shows<br />
the impedance <strong>of</strong> the PS-TFSI gel at lithium metal at 80°C<br />
which is an order <strong>of</strong> magnitude less than the polyether<br />
material. Even at 25°C the impedance is only 200 ohm.cm 2<br />
which indicates that these single-ion conductor materials<br />
are now very close to being practical.<br />
More important is the performance <strong>of</strong> these materials<br />
when used as binders in composite electrodes. Figure V - 141<br />
shows the impedance <strong>of</strong> half cells <strong>of</strong> LiFePO 4 one with the<br />
single-ion conductor separator and binder and one with a<br />
conventional binary salt electrolyte. Figure V - 142 shows the<br />
discharge capacities <strong>of</strong> these two cells as a function <strong>of</strong> rate.<br />
Figure V - 142 shows the much better capacity at high<br />
rates <strong>of</strong> the single-ion conductor binder which is expected<br />
due to the lack <strong>of</strong> concentration polarization. The stability<br />
<strong>of</strong> the PS-TFSI SIC gel at high voltages is illustrated in<br />
Figure V - 143, where it can be seen that the electrolyte<br />
system is stable to at least 4.5V. This indicates the material<br />
will be stable with the spinel –type cathodes and tests on<br />
this stability are under way.<br />
The interfacial impedance <strong>of</strong> these SIC materials is<br />
still high but maybe reduced by functionalization <strong>of</strong> the<br />
surfaces in composite electrodes as illustrated in Figure V -<br />
136 (b). Very encouraging results have already been<br />
obtained that will merit a patent application in due course.<br />
The results will be reported on in future.<br />
Energy Storage R &D 600 FY 2011 Annual Progress Report