V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Whittingham – Binghamton U.<br />
V.B.5 Synthesis and Characterization <strong>of</strong> Substituted Olivines and Mn Oxides (Binghamton U.)<br />
materials. To meet the DOE cost targets, we are looking at<br />
reducing high-cost components and for power and energy<br />
targets at modifying the chemical composition and<br />
morphology <strong>of</strong> the cathode compounds.<br />
Approach<br />
Our cathode approach is to place emphasis on low<br />
cost materials, predominantly oxides and phosphates, both<br />
pure and modified with other transition metals, using a<br />
range <strong>of</strong> practical synthesis approaches. These materials<br />
will be synthesized, and characterized both structurally,<br />
including defects and morphology, and for thermal and<br />
chemical stability. All will be evaluated electrochemically<br />
in a range <strong>of</strong> cell configurations.<br />
For the modified layered dioxides, we are determining<br />
the role <strong>of</strong> each <strong>of</strong> the transition metals, with the goal <strong>of</strong><br />
minimizing expensive components such as cobalt. To that<br />
end, we are studying the layered compositions,<br />
LiNi y Mn y Co 1-2y O 2 , with a close to stoichiometric Li to<br />
transition metal ratio and comparing them to the Li-rich<br />
Mn-rich compounds.<br />
We are also searching for new classes <strong>of</strong> materials<br />
that might react with more than one lithium ion per redox<br />
center. Phosphates are one area for our search and<br />
vanadium containing materials another area.<br />
Results<br />
Layered Transition Metal Oxides. We formed a<br />
range <strong>of</strong> transition metal oxides <strong>of</strong> formula LiNi y Mn y Co 1<br />
2yO 2 to determine the optimum composition for both<br />
energy density and power density. The voltage/capacity<br />
curves were determined and found to depend little on the<br />
chemical composition except at low values <strong>of</strong> the lithium<br />
content, when the redox <strong>of</strong> Co becomes involved and<br />
pushed the potential higher. The lower cobalt material with<br />
y = 0.4 showed as good electrochemical behavior as the<br />
commonly used LiNi 1/3 Mn 1/3 Co 1/3 O 2 material, except at the<br />
highest discharge rates. In collaboration with C. Ban and<br />
A. Dillon at NREL, the rate behavior was determined and<br />
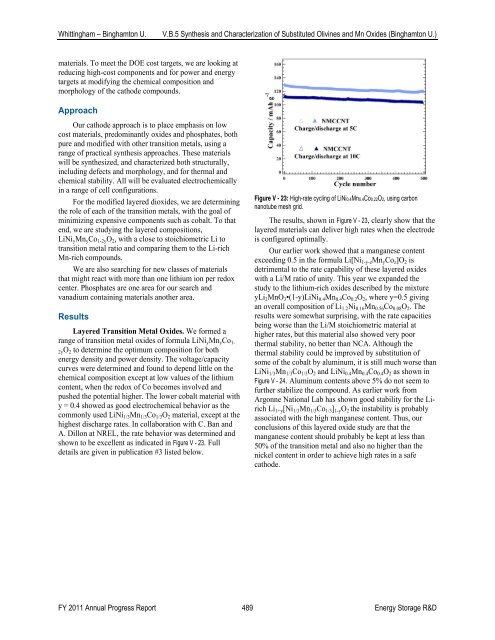
shown to be excellent as indicated in Figure V - 23. Full<br />
details are given in publication #3 listed below.<br />
Figure V - 23: High-rate cycling <strong>of</strong> LiNi0.4Mn0.4Co0.22O2, using carbon<br />
nanotube mesh grid.<br />
The results, shown in Figure V - 23, clearly show that the<br />
layered materials can deliver high rates when the electrode<br />
is configured optimally.<br />
Our earlier work showed that a manganese content<br />
exceeding 0.5 in the formula Li[Ni 1-y-z Mn y Co z ]O 2 is<br />
detrimental to the rate capability <strong>of</strong> these layered oxides<br />
with a Li/M ratio <strong>of</strong> unity. This year we expanded the<br />
study to the lithium-rich oxides described by the mixture<br />
yLi 2 MnO 3 •(1-y)LiNi 0.4 Mn 0.4 Co 0.2 O 2 , where y=0.5 giving<br />
an overall composition <strong>of</strong> Li 1.2 Ni 0.16 Mn 0.56 Co 0.08 O 2 . The<br />
results were somewhat surprising, with the rate capacities<br />
being worse than the Li/M stoichiometric material at<br />
higher rates, but this material also showed very poor<br />
thermal stability, no better than NCA. Although the<br />
thermal stability could be improved by substitution <strong>of</strong><br />
some <strong>of</strong> the cobalt by aluminum, it is still much worse than<br />
LiNi 1/3 Mn 1/3 Co 1/3 O 2 and LiNi 0.4 Mn 0.4 Co 0.4 O 2 as shown in<br />
Figure V - 24. Aluminum contents above 5% do not seem to<br />
further stabilize the compound. As earlier work from<br />
Argonne National Lab has shown good stability for the Lirich<br />
Li 1+y [Ni 1/3 Mn 1/3 Co 1/3 ] 1-y O 2 the instability is probably<br />
associated with the high manganese content. Thus, our<br />
conclusions <strong>of</strong> this layered oxide study are that the<br />
manganese content should probably be kept at less than<br />
50% <strong>of</strong> the transition metal and also no higher than the<br />
nickel content in order to achieve high rates in a safe<br />
cathode.<br />
FY 2011 Annual Progress Report 489 Energy Storage R&D