V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Lucht – URI<br />
V.D.7 Development <strong>of</strong> Electrolytes for Lithium-ion Batteries (URI)<br />
Results<br />
Development <strong>of</strong> Cathode Film Forming Additives.<br />
An investigation <strong>of</strong> the reactions <strong>of</strong> electrolyte with the<br />
surface <strong>of</strong> LiNi 0.5 Mn 1.5 O 4 cathode materials cycled to 4.9<br />
V vs Li or stored at elevated temperature (55°C for two<br />
weeks) has been conducted to develop a better<br />
understanding <strong>of</strong> the sources <strong>of</strong> performance fade. In<br />
order to investigate the difference in electrolyte oxidation<br />
on LiNi 0.5 Mn 1.5 O 4 vs Pt, electrodes were stored at 4.75 and<br />
5.30 V vs Li for several days. After subtraction <strong>of</strong> the<br />
current associated with lithium extraction from<br />
LiNi 0.5 Mn 1.5 O 4 the residual currents are very similar<br />
suggesting that the oxidation reactions are similar for<br />
LiNi 0.5 Mn 1.5 O 4 and Pt (Figure V - 161). Surface analysis<br />
suggests that the surface species on LiNi 0.5 Mn 1.5 O 4 and Pt<br />
are very similar. Thus, electrolyte oxidation reactions<br />
apper to be largely independent <strong>of</strong> the electrode structure.<br />
smaller impedance suggests thinner surface films. Related<br />
investigations are also being conducted on novel Lewis<br />
basic additives to inhibit Mn leaching form the cathode<br />
and provide similar performance improvements.<br />
Discharge Capacity, mAh/g<br />
200<br />
180<br />
160<br />
140<br />
120<br />
100<br />
STD ELECTROLYTE<br />
1% LiBOB<br />
0.25% LiBOB<br />
Elevated temperature storage experuiments suggest that 80<br />
0 2 4 6 8 10 12 14<br />
the electrolyte reacts with the cathode surface and Mn<br />
Cycle Number<br />
dissolution is a problem for LiPF 6 electrolytes.<br />
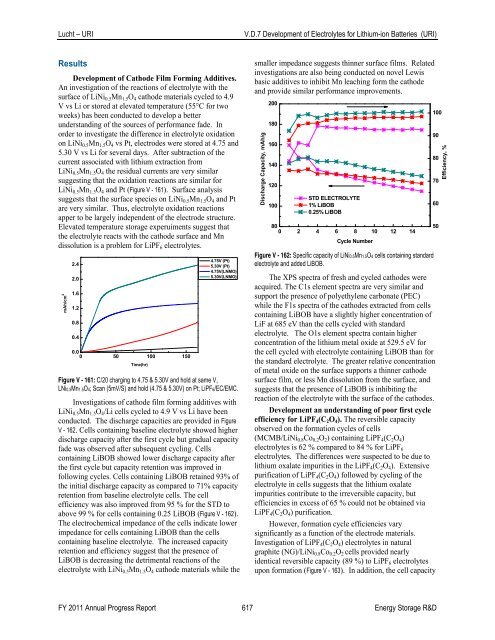
Figure V - 162: Specific capacity <strong>of</strong> LiNi0.5Mn1.5O4 cells containing standard<br />
2.4<br />
4.75V (Pt)<br />
5.30V (Pt) electrolyte and added LiBOB.<br />
4.75V(LNMO)<br />
mAh/cm 2<br />
2.0<br />
1.6<br />
1.2<br />
0.8<br />
0.4<br />
0.0<br />
0 50 100 150<br />
Time(hr)<br />
5.30V(LNMO)<br />
Figure V - 161: C/20 charging to 4.75 & 5.30V and hold at same V,<br />
LNi0.5Mn1.5O4; Scan (5mV/S) and hold (4.75 & 5.30V) on Pt; LiPF6/EC/EMC.<br />
Investigations <strong>of</strong> cathode film forming additives with<br />
LiNi 0.5 Mn 1.5 O 4 /Li cells cycled to 4.9 V vs Li have been<br />
conducted. The discharge capacities are provided in Figure<br />
V - 162. Cells containing baseline electrolyte showed higher<br />
discharge capacity after the first cycle but gradual capacity<br />
fade was observed after subsequent cycling. Cells<br />
containing LiBOB showed lower discharge capacity after<br />
the first cycle but capacity retention was improved in<br />
following cycles. Cells containing LiBOB retained 93% <strong>of</strong><br />
the initial discharge capacity as compared to 71% capacity<br />
retention from baseline electrolyte cells. The cell<br />
efficiency was also improved from 95 % for the STD to<br />
above 99 % for cells containing 0.25 LiBOB (Figure V - 162).<br />
The electrochemical impedance <strong>of</strong> the cells indicate lower<br />
impedance for cells containing LiBOB than the cells<br />
containing baseline electrolyte. The increased capacity<br />
retention and efficiency suggest that the presence <strong>of</strong><br />
LiBOB is decreasing the detrimental reactions <strong>of</strong> the<br />
electrolyte with LiNi 0.5 Mn 1.5 O 4 cathode materials while the<br />
The XPS spectra <strong>of</strong> fresh and cycled cathodes were<br />
acquired. The C1s element spectra are very similar and<br />
support the presence <strong>of</strong> polyethylene carbonate (PEC)<br />
while the F1s spectra <strong>of</strong> the cathodes extracted from cells<br />
containing LiBOB have a slightly higher concentration <strong>of</strong><br />
LiF at 685 eV than the cells cycled with standard<br />
electrolyte. The O1s element spectra contain higher<br />
concentration <strong>of</strong> the lithium metal oxide at 529.5 eV for<br />
the cell cycled with electrolyte containing LiBOB than for<br />
the standard electrolyte. The greater relative concentration<br />
<strong>of</strong> metal oxide on the surface supports a thinner cathode<br />
surface film, or less Mn dissolution from the surface, and<br />
suggests that the presence <strong>of</strong> LiBOB is inhibiting the<br />
reaction <strong>of</strong> the electrolyte with the surface <strong>of</strong> the cathodes.<br />
Development an understanding <strong>of</strong> poor first cycle<br />
efficiency for LiPF 4 (C 2 O 4 ). The reversible capacity<br />
observed on the formation cycles <strong>of</strong> cells<br />
(MCMB/LiNi 0.8 Co 0.2 O 2 ) containing LiPF 4 (C 2 O 4 )<br />
electrolytes is 62 % compared to 84 % for LiPF 6<br />
electrolytes. The differences were suspected to be due to<br />
lithium oxalate impurities in the LiPF 4 (C 2 O 4 ). Extensive<br />
purification <strong>of</strong> LiPF 4 (C 2 O 4 ) followed by cycling <strong>of</strong> the<br />
electrolyte in cells suggests that the lithium oxalate<br />
impurities contribute to the irreversible capacity, but<br />
efficiencies in excess <strong>of</strong> 65 % could not be obtained via<br />
LiPF 4 (C 2 O 4 ) purification.<br />
However, formation cycle efficiencies vary<br />
significantly as a function <strong>of</strong> the electrode materials.<br />
Investigation <strong>of</strong> LiPF 4 (C 2 O 4 ) electrolytes in natural<br />
graphite (NG)/LiNi 0.8 Co 0.2 O 2 cells provided nearly<br />
identical reversible capacity (89 %) to LiPF 6 electrolytes<br />
upon formation (Figure V - 163). In addition, the cell capacity<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
Efficiency, %<br />
FY 2011 Annual Progress Report 617 Energy Storage R&D