V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
V.E.7 Predicting/Understanding New Li-ion Materials Using Ab Initio Atomistic Computational Methods (LBNL)<br />
Persson – LBNL<br />
results in a different response <strong>of</strong> the material when<br />
synthesized under different conditions.<br />
Approach<br />
The Persson group uses atomistic modeling to study<br />
the relevant thermodynamic and kinetic processes. The<br />
calculations are performed on the Lawrencium cluster at<br />
LBNL and at NERSC. In the case <strong>of</strong> the high voltage<br />
spinel we have used first-principles zero-temperature<br />
calculations and a coupled cluster expansion to establish<br />
the relationship between the cation order and the<br />
electrochemical signature <strong>of</strong> the materials.<br />
For the investigation on the Li-carbon system we are<br />
using a combination <strong>of</strong> DFT to describe the low<br />
temperature characteristics <strong>of</strong> the material as well as<br />
statistical mechanics to calculate the phase diagram and<br />
the Li chemical diffusivity and phenomenological models<br />
to capture the van der Waals interactions.<br />
Results<br />
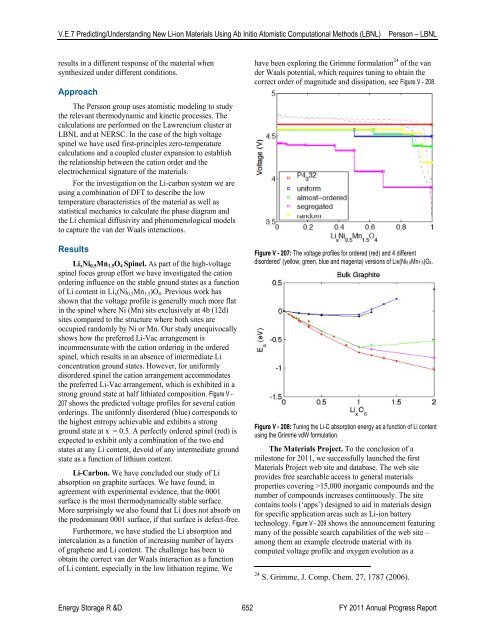
Li x Ni 0.5 Mn 1.5 O 4 Spinel. As part <strong>of</strong> the high-voltage<br />
spinel focus group effort we have investigated the cation<br />
ordering influence on the stable ground states as a function<br />
<strong>of</strong> Li content in Li x (Ni 0.5 Mn 1.5 )O 4 . Previous work has<br />
shown that the voltage pr<strong>of</strong>ile is generally much more flat<br />
in the spinel where Ni (Mn) sits exclusively at 4b (12d)<br />
sites compared to the structure where both sites are<br />
occupied randomly by Ni or Mn. Our study unequivocally<br />
shows how the preferred Li-Vac arrangement is<br />
incommensurate with the cation ordering in the ordered<br />
spinel, which results in an absence <strong>of</strong> intermediate Li<br />
concentration ground states. However, for uniformly<br />
disordered spinel the cation arrangement accommodates<br />
the preferred Li-Vac arrangement, which is exhibited in a<br />
strong ground state at half lithiated composition. Figure V -<br />
207 shows the predicted voltage pr<strong>of</strong>iles for several cation<br />
orderings. The uniformly disordered (blue) corresponds to<br />
the highest entropy achievable and exhibits a strong<br />
ground state at x = 0.5. A perfectly ordered spinel (red) is<br />
expected to exhibit only a combination <strong>of</strong> the two end<br />
states at any Li content, devoid <strong>of</strong> any intermediate ground<br />
state as a function <strong>of</strong> lithium content.<br />
Li-Carbon. We have concluded our study <strong>of</strong> Li<br />
absorption on graphite surfaces. We have found, in<br />
agreement with experimental evidence, that the 0001<br />
surface is the most thermodynamically stable surface.<br />
More surprisingly we also found that Li does not absorb on<br />
the predominant 0001 surface, if that surface is defect-free.<br />
Furthermore, we have studied the Li absorption and<br />
intercalation as a function <strong>of</strong> increasing number <strong>of</strong> layers<br />
<strong>of</strong> graphene and Li content. The challenge has been to<br />
obtain the correct van der Waals interaction as a function<br />
<strong>of</strong> Li content, especially in the low lithiation regime. We<br />
have been exploring the Grimme formulation 24 <strong>of</strong> the van<br />
der Waals potential, which requires tuning to obtain the<br />
correct order <strong>of</strong> magnitude and dissipation, see Figure V - 208.<br />
Figure V - 207: The voltage pr<strong>of</strong>iles for ordered (red) and 4 different<br />
disordered’ (yellow, green, blue and magenta) versions <strong>of</strong> Lix(Ni0.5Mn1.5)O4.<br />
Figure V - 208: Tuning the Li-C absorption energy as a function <strong>of</strong> Li content<br />
using the Grimme vdW formulation.<br />
The Materials Project. To the conclusion <strong>of</strong> a<br />
milestone for 2011, we successfully launched the first<br />
Materials Project web site and database. The web site<br />
provides free searchable access to general materials<br />
properties covering >15,000 inorganic compounds and the<br />
number <strong>of</strong> compounds increases continuously. The site<br />
contains tools (‘apps’) designed to aid in materials design<br />
for specific application areas such as Li-ion battery<br />
technology. Figure V - 209 shows the announcement featuring<br />
many <strong>of</strong> the possible search capabilities <strong>of</strong> the web site –<br />
among them an example electrode material with its<br />
computed voltage pr<strong>of</strong>ile and oxygen evolution as a<br />
24 S. Grimme, J. Comp. Chem. 27, 1787 (2006).<br />
Energy Storage R &D 652 FY 2011 Annual Progress Report