V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
V.B.8 Characterization <strong>of</strong> New Cathode Materials and Studies <strong>of</strong> Li-Air Batteries (BNL, U. Mass)<br />
Nam – U. Mass, Yang – BNL<br />
developing new diagnostic tools to investigate battery<br />
materials both in situ and ex situ, and then applies these to<br />
explain the relationships between structure and function<br />
for new materials.<br />
Connected with pump<br />
Approach<br />
1. In situ XAS and XRD studies <strong>of</strong> new electrode<br />
materials such as LiFe 1-y Mn y PO 4 and high capacity<br />
Li 1.2 Ni 0.2 Mn 0.6 O 2 during electrochemical cycling to<br />
carry out the diagnostic studies to improve the energy<br />
density and cycle life <strong>of</strong> Li-ion batteries.<br />
2. S<strong>of</strong>t XAS on the L-edges <strong>of</strong> Mn and Ni to distinguish<br />
the difference between the surface and the bulk<br />
structural changes caused by charge-discharge cycling<br />
for new cathode materials such as Li 1.2 Ni 0.2 Mn 0.6 O 2 .<br />
3. In situ and ex situ transmission electron microscopy<br />
(TEM) coupled with selected area electron diffraction<br />
(SAED) to study the structural changes <strong>of</strong> electrode<br />
materials with high location specification and spatial<br />
resolution.<br />
4. Electrochemical studies <strong>of</strong> GDE for Li-air batteries.<br />
5. Improve the performance <strong>of</strong> the GDE by surface<br />
modification.<br />
6. Design and synthesis <strong>of</strong> new electrolyte system with<br />
capability to dissolve Li 2 O and Li 2 O 2 , and/or high<br />
solubility <strong>of</strong> O 2 for Li-air batteries.<br />
Results<br />
Continued the in situ x-ray absorption and time<br />
resolved XRD studies <strong>of</strong> G2 and G3 cathode materials<br />
during heating<br />
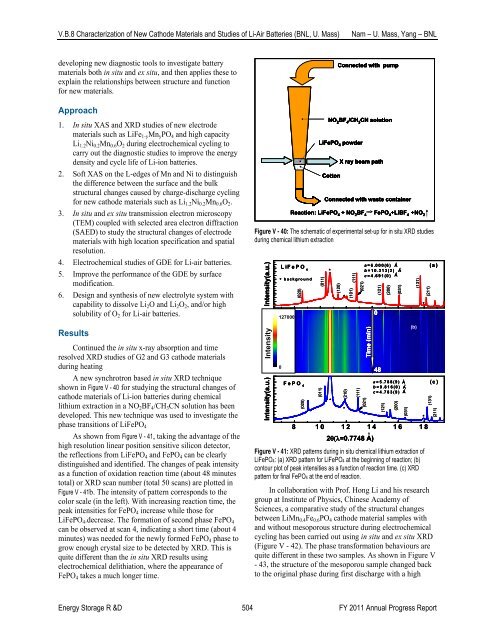
A new synchrotron based in situ XRD technique<br />
shown in Figure V - 40 for studying the structural changes <strong>of</strong><br />
cathode materials <strong>of</strong> Li-ion batteries during chemical<br />
lithium extraction in a NO 2 BF 4 /CH 3 CN solution has been<br />
developed. This new technique was used to investigate the<br />
phase transitions <strong>of</strong> LiFePO 4<br />
As shown from Figure V - 41, taking the advantage <strong>of</strong> the<br />
high resolution linear position sensitive silicon detector,<br />
the reflections from LiFePO 4 and FePO 4 can be clearly<br />
distinguished and identified. The changes <strong>of</strong> peak intensity<br />
as a function <strong>of</strong> oxidation reaction time (about 48 minutes<br />
total) or XRD scan number (total 50 scans) are plotted in<br />
Figure V - 41b. The intensity <strong>of</strong> pattern corresponds to the<br />
color scale (in the left). With increasing reaction time, the<br />
peak intensities for FePO 4 increase while those for<br />
LiFePO 4 .decrease. The formation <strong>of</strong> second phase FePO 4<br />
can be observed at scan 4, indicating a short time (about 4<br />
minutes) was needed for the newly formed FePO 4 phase to<br />
grow enough crystal size to be detected by XRD. This is<br />
quite different than the in situ XRD results using<br />
electrochemical delithiation, where the appearance <strong>of</strong><br />
FePO 4 takes a much longer time.<br />
Figure V - 40: The schematic <strong>of</strong> experimental set-up for in situ XRD studies<br />
during chemical lithium extraction<br />
Intensity(a.u.)<br />
Intensity<br />
Intensity(a.u.)<br />
127000<br />
0<br />
NO 2 BF 4 /CH 3 CN solution<br />
LiFePO 4 powder<br />
Cotton<br />
X ray beam path<br />
Connected with waste container<br />
Reaction: LiFePO 4<br />
+ NO 2<br />
BF<br />
---> 4 FePO 4 +LiBF 4 +NO 2<br />
LiFePO 4<br />
*<br />
background<br />
(020)<br />
FePO 4<br />
(020)<br />
(011)<br />
(011)<br />
*<br />
*<br />
(120)<br />
*<br />
(210)<br />
*<br />
(111)<br />
(101)<br />
(111)<br />
(021)<br />
a =6.000(6)<br />
A<br />
b =10.313(2)<br />
c =4.691(0)<br />
Time (min)<br />
(021)<br />
(121)<br />
0<br />
48<br />
(200)<br />
o<br />
A o Ao<br />
(031)<br />
a =5.788(9)<br />
A o b =9.816(0)<br />
A o<br />
c =4.783(9)<br />
A o<br />
(121)<br />
(200)<br />
(031)<br />
(131)<br />
(b)<br />
8 1 0 1 2 1 4 1 6 1 8<br />
2<br />
=0.774<br />
.7748 A)<br />
Figure V - 41: XRD patterns during in situ chemical lithium extraction <strong>of</strong><br />
LiFePO4: (a) XRD pattern for LiFePO4 at the beginning <strong>of</strong> reaction; (b)<br />
contour plot <strong>of</strong> peak intensities as a function <strong>of</strong> reaction time. (c) XRD<br />
pattern for final FePO4 at the end <strong>of</strong> reaction.<br />
In collaboration with Pr<strong>of</strong>. Hong Li and his research<br />
group at Institute <strong>of</strong> Physics, Chinese Academy <strong>of</strong><br />
Sciences, a comparative study <strong>of</strong> the structural changes<br />
between LiMn 0.4 Fe 0.6 PO 4 cathode material samples with<br />
and without mesoporous structure during electrochemical<br />
cycling has been carried out using in situ and ex situ XRD<br />
(Figure V - 42). The phase transformation behaviours are<br />
quite different in these two samples. As shown in Figure V<br />
- 43, the structure <strong>of</strong> the mesoporou sample changed back<br />
to the original phase during first discharge with a high<br />
o<br />
*<br />
*<br />
(211)<br />
(a)<br />
(c)<br />
(131)<br />
(211)<br />
Energy Storage R &D 504 FY 2011 Annual Progress Report