V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
V. Focused Fundamental Research - EERE - U.S. Department of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
V.B.8 Characterization <strong>of</strong> New Cathode Materials and Studies <strong>of</strong> Li-Air Batteries (BNL, U. Mass)<br />
Nam – U. Mass, Yang – BNL<br />
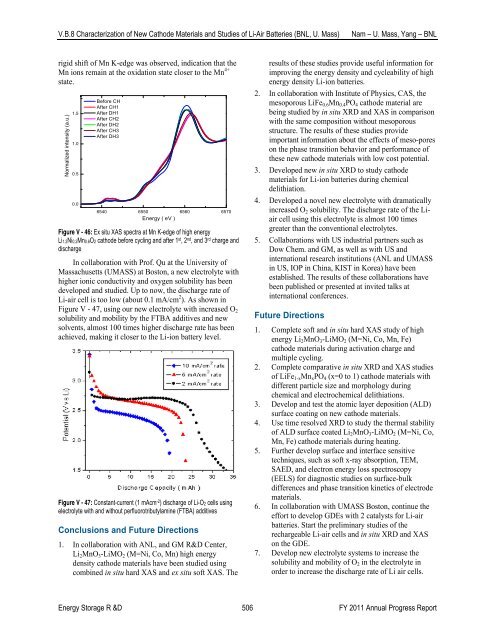
rigid shift <strong>of</strong> Mn K-edge was observed, indication that the<br />
Mn ions remain at the oxidation state closer to the Mn 4+<br />
state.<br />
Normalized intensity (a.u.)<br />
1.5<br />
1.0<br />
0.5<br />
0.0<br />
Before CH<br />
After CH1<br />
After DH1<br />
After CH2<br />
After DH2<br />
After CH3<br />
After DH3<br />
6540 6550 6560 6570<br />
Energy ( eV )<br />
Figure V - 46: Ex situ XAS spectra at Mn K-edge <strong>of</strong> high energy<br />
Li1.2Ni0.2Mn0.6O2 cathode before cycling and after 1 st , 2 nd , and 3 rd charge and<br />
discharge<br />
In collaboration with Pr<strong>of</strong>. Qu at the University <strong>of</strong><br />
Massachusetts (UMASS) at Boston, a new electrolyte with<br />
higher ionic conductivity and oxygen solubility has been<br />
developed and studied. Up to now, the discharge rate <strong>of</strong><br />
Li-air cell is too low (about 0.1 mA/cm 2 ). As shown in<br />
Figure V - 47, using our new electrolyte with increased O 2<br />
solubility and mobility by the FTBA additives and new<br />
solvents, almost 100 times higher discharge rate has been<br />
achieved, making it closer to the Li-ion battery level.<br />
Figure V - 47: Constant-current (1 mAcm -2 ) discharge <strong>of</strong> Li-O2 cells using<br />
electrolyte with and without perfluorotributylamine (FTBA) additives<br />
Conclusions and Future Directions<br />
1. In collaboration with ANL, and GM R&D Center,<br />
Li 2 MnO 3 -LiMO 2 (M=Ni, Co, Mn) high energy<br />
density cathode materials have been studied using<br />
combined in situ hard XAS and ex situ s<strong>of</strong>t XAS. The<br />
results <strong>of</strong> these studies provide useful information for<br />
improving the energy density and cycleability <strong>of</strong> high<br />
energy density Li-ion batteries.<br />
2. In collaboration with Institute <strong>of</strong> Physics, CAS, the<br />
mesoporous LiFe 0.6 Mn 0.4 PO 4 cathode material are<br />
being studied by in situ XRD and XAS in comparison<br />
with the same composition without mesoporous<br />
structure. The results <strong>of</strong> these studies provide<br />
important information about the effects <strong>of</strong> meso-pores<br />
on the phase transition behavior and performance <strong>of</strong><br />
these new cathode materials with low cost potential.<br />
3. Developed new in situ XRD to study cathode<br />
materials for Li-ion batteries during chemical<br />
delithiation.<br />
4. Developed a novel new electrolyte with dramatically<br />
increased O 2 solubility. The discharge rate <strong>of</strong> the Liair<br />
cell using this electrolyte is almost 100 times<br />
greater than the conventional electrolytes.<br />
5. Collaborations with US industrial partners such as<br />
Dow Chem. and GM, as well as with US and<br />
international research institutions (ANL and UMASS<br />
in US, IOP in China, KIST in Korea) have been<br />
established. The results <strong>of</strong> these collaborations have<br />
been published or presented at invited talks at<br />
international conferences.<br />
Future Directions<br />
1. Complete s<strong>of</strong>t and in situ hard XAS study <strong>of</strong> high<br />
energy Li 2 MnO 3 -LiMO 2 (M=Ni, Co, Mn, Fe)<br />
cathode materials during activation charge and<br />
multiple cycling.<br />
2. Complete comparative in situ XRD and XAS studies<br />
<strong>of</strong> LiFe 1-x Mn x PO 4 (x=0 to 1) cathode materials with<br />
different particle size and morphology during<br />
chemical and electrochemical delithiations.<br />
3. Develop and test the atomic layer deposition (ALD)<br />
surface coating on new cathode materials.<br />
4. Use time resolved XRD to study the thermal stability<br />
<strong>of</strong> ALD surface coated Li 2 MnO 3 -LiMO 2 (M=Ni, Co,<br />
Mn, Fe) cathode materials during heating.<br />
5. Further develop surface and interface sensitive<br />
techniques, such as s<strong>of</strong>t x-ray absorption, TEM,<br />
SAED, and electron energy loss spectroscopy<br />
(EELS) for diagnostic studies on surface-bulk<br />
differences and phase transition kinetics <strong>of</strong> electrode<br />
materials.<br />
6. In collaboration with UMASS Boston, continue the<br />
effort to develop GDEs with 2 catalysts for Li-air<br />
batteries. Start the preliminary studies <strong>of</strong> the<br />
rechargeable Li-air cells and in situ XRD and XAS<br />
on the GDE.<br />
7. Develop new electrolyte systems to increase the<br />
solubility and mobility <strong>of</strong> O 2 in the electrolyte in<br />
order to increase the discharge rate <strong>of</strong> Li air cells.<br />
Energy Storage R &D 506 FY 2011 Annual Progress Report