- Page 3:
Technical Guidelines forIntegrated
- Page 6 and 7:
3.3.1 Review the updated charts, ta
- Page 8 and 9:
7.1.2 Monitor timeliness and comple
- Page 10 and 11:
List of TablesTable 1: Surveillance
- Page 13:
AcronymsAFROAFPAIDSCBSCBSWCDCDHMTE-
- Page 18 and 19:
An ill person presents to medical a
- Page 20 and 21:
How are surveillance functions desc
- Page 22 and 23:
4.0 Investigate** 5.0 Respond 6.0 P
- Page 24 and 25:
National4.0 Investigate** 5.0 Respo
- Page 26 and 27:
In general, the health of Ghanaians
- Page 28 and 29:
How does Ghana’s MOH/GHS support
- Page 30 and 31:
1.0 Identify Cases of Priority Dise
- Page 32 and 33:
1.2 Improve district procedures for
- Page 35 and 36:
Section 2Report Priority Diseases a
- Page 37 and 38:
Name of diseaseRoutine summary repo
- Page 39 and 40:
Identify and train CBS volunteers o
- Page 41:
Clinicians, ward nurses or other re
- Page 44 and 45:
3.0 Analyse DataAnalysing trends of
- Page 46 and 47:
3. Label all the rows and columns,
- Page 48 and 49:
Figure 4: Meningitis cases from Bol
- Page 50 and 51:
The first step in analysing data by
- Page 52 and 53:
3.2.3.3 Calculate case fatality rat
- Page 54 and 55:
Table 6: Alert and epidemic/action
- Page 56 and 57:
3.4 Use the analysis results to imp
- Page 58 and 59:
4.0 Investigate Reported Outbreaks
- Page 60 and 61:
4.4 Prepare to conduct an investiga
- Page 62 and 63:
Review laboratory results with the
- Page 64 and 65:
Draw a histogram representing the c
- Page 66 and 67:
If the shape of the curve suddenly
- Page 68 and 69:
5.0 Prepare and Respond to Outbreak
- Page 70 and 71:
Periodically review and update othe
- Page 72 and 73:
3. Select appropriate communication
- Page 74 and 75:
Improve safe disposal of human wast
- Page 76 and 77:
Table 7: Daily water needs per pers
- Page 78 and 79:
Recommendations for improving epide
- Page 80 and 81:
6.0 Provide FeedbackData are report
- Page 82 and 83:
The newsletter can be produced simp
- Page 84 and 85:
7.0 Evaluate and Improve Surveillan
- Page 86 and 87:
Table 8 lists possible indicators t
- Page 88 and 89:
Priority diseases are recorded in t
- Page 90 and 91:
Indicators for measuring quality of
- Page 93 and 94:
Section 8Community-based Surveillan
- Page 95 and 96:
members to take action in the event
- Page 97 and 98:
Participate in information dissemin
- Page 99 and 100:
Section 9Summary Guidelines for Spe
- Page 101 and 102:
Analyse and interpretdataReferenceT
- Page 103 and 104:
Buruli UlcerBackground Buruli ulce
- Page 105 and 106:
Cholera may cause severe dehydratio
- Page 107 and 108:
Respond to epidemicthresholdIf a su
- Page 109 and 110:
In Ghana, the disease was endemic i
- Page 111 and 112:
ReferenceA guide to eliminating lep
- Page 113 and 114:
Promote environmental sanitation: c
- Page 115 and 116:
Surveillance goal Use a rapid late
- Page 117 and 118:
OnchocerciasisBackground Filarial
- Page 119 and 120:
Poliomyelitis (Acute flaccid paraly
- Page 122 and 123: Public health action Conduct activ
- Page 124 and 125: The global HIV pandemic has been a
- Page 126 and 127: Respond toepidemicAnalyse and inter
- Page 128 and 129: Public health action Case finding
- Page 131 and 132: Annex 1Using Assessment Results to
- Page 133: Tally, compile and report summary t
- Page 136 and 137: Diseases of special public health f
- Page 138 and 139: Pneumonia in childrenless than 5 ye
- Page 140 and 141: Other diseases of public health imp
- Page 143 and 144: Annex 5Laboratory Tests for Confirm
- Page 145 and 146: Suspected disease orconditionCholer
- Page 147 and 148: Suspected disease orconditionDiagno
- Page 149 and 150: Suspected disease orconditionDiagno
- Page 151 and 152: Suspected disease orconditionDiagno
- Page 153: Annex 6List of Laboratories for Con
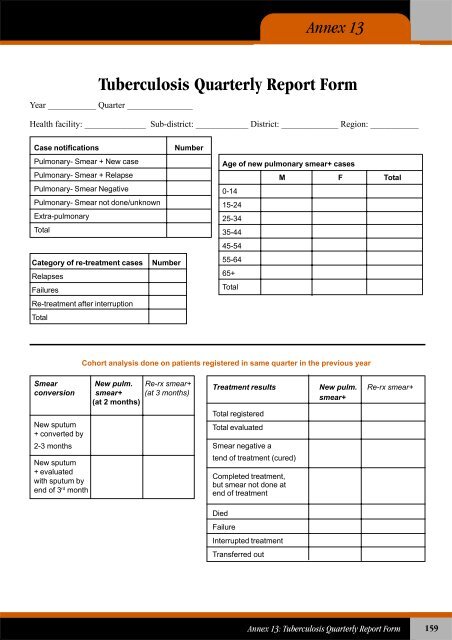
- Page 157 and 158: Annex 8Case-based Surveillance Repo
- Page 159 and 160: If laboratory specimen collectedFor
- Page 161: 16. When the investigation of the c
- Page 164 and 165: (For reporting from health facility
- Page 166 and 167: 13. For vaccine-preventable disease
- Page 169 and 170: Annex 11Total Number of Patients Se
- Page 171: Annex 12Year ___________ Quarter __
- Page 175 and 176: Annex 14Cased-based Surveillance Re
- Page 177: G. Treatment Outcomes25. Healed wit
- Page 180 and 181: Year: _____________________ Month:
- Page 183: Annex 16Quarterly Report on New and
- Page 187: Annex 18Sample Grid for Time Analys
- Page 190 and 191: Comparing inpatient and outpatient
- Page 192 and 193: No. of casesactually identified(7)D
- Page 195 and 196: Annex 21Case Investigation Form - N
- Page 197 and 198: Annex 22Case Investigation Form - A
- Page 199 and 200: Annex 23Case Investigation Form - G
- Page 201 and 202: Annex 23: Case Investigation Form -
- Page 203: Annex 24Checklist of Laboratory Sup
- Page 206 and 207: Antibiotics recommended for treatme
- Page 208 and 209: Reassess the patient after the firs
- Page 210 and 211: Other recommended antibiotics to tr
- Page 213 and 214: Annex 27Conducting a Register Revie
- Page 215 and 216: Annex 28Sample Messages for Communi
- Page 217 and 218: Clean drinking water and storageCom
- Page 219: Annex 29Planning an Emergency Immun
- Page 222 and 223:
6. If the vaccine requires a diluen
- Page 225 and 226:
Annex 32District Outbreak Report Fo
- Page 227 and 228:
Annex 33Self-evaluation of the Time
- Page 229:
Annex 34Record of Reports ReceivedD
- Page 232 and 233:
Health facility:___________________
- Page 234 and 235:
Technical Guidelines for Integrated
- Page 236:
SupervisionTrainingResources andper