Untitled - Technische Universiteit Eindhoven
Untitled - Technische Universiteit Eindhoven
Untitled - Technische Universiteit Eindhoven
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
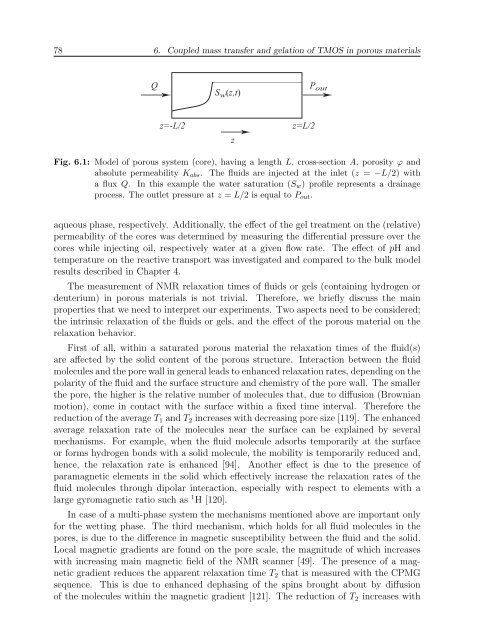
78 6. Coupled mass transfer and gelation of TMOS in porous materialsQS w (z,t)P outz=-L/2zz=L/2Fig. 6.1: Model of porous system (core), having a length L, cross-section A, porosity ϕ andabsolute permeability K abs . The fluids are injected at the inlet (z = −L/2) witha flux Q. In this example the water saturation (S w ) profile represents a drainageprocess. The outlet pressure at z = L/2 is equal to P out .aqueous phase, respectively. Additionally, the effect of the gel treatment on the (relative)permeability of the cores was determined by measuring the differential pressure over thecores while injecting oil, respectively water at a given flow rate. The effect of pH andtemperature on the reactive transport was investigated and compared to the bulk modelresults described in Chapter 4.The measurement of NMR relaxation times of fluids or gels (containing hydrogen ordeuterium) in porous materials is not trivial. Therefore, we briefly discuss the mainproperties that we need to interpret our experiments. Two aspects need to be considered;the intrinsic relaxation of the fluids or gels, and the effect of the porous material on therelaxation behavior.First of all, within a saturated porous material the relaxation times of the fluid(s)are affected by the solid content of the porous structure. Interaction between the fluidmolecules and the pore wall in general leads to enhanced relaxation rates, depending on thepolarity of the fluid and the surface structure and chemistry of the pore wall. The smallerthe pore, the higher is the relative number of molecules that, due to diffusion (Brownianmotion), come in contact with the surface within a fixed time interval. Therefore thereduction of the average T 1 and T 2 increases with decreasing pore size [119]. The enhancedaverage relaxation rate of the molecules near the surface can be explained by severalmechanisms. For example, when the fluid molecule adsorbs temporarily at the surfaceor forms hydrogen bonds with a solid molecule, the mobility is temporarily reduced and,hence, the relaxation rate is enhanced [94]. Another effect is due to the presence ofparamagnetic elements in the solid which effectively increase the relaxation rates of thefluid molecules through dipolar interaction, especially with respect to elements with alarge gyromagnetic ratio such as 1 H [120].In case of a multi-phase system the mechanisms mentioned above are important onlyfor the wetting phase. The third mechanism, which holds for all fluid molecules in thepores, is due to the difference in magnetic susceptibility between the fluid and the solid.Local magnetic gradients are found on the pore scale, the magnitude of which increaseswith increasing main magnetic field of the NMR scanner [49]. The presence of a magneticgradient reduces the apparent relaxation time T 2 that is measured with the CPMGsequence. This is due to enhanced dephasing of the spins brought about by diffusionof the molecules within the magnetic gradient [121]. The reduction of T 2 increases with