Table 6.5.1NSAIDDosemgÆkg )1IntervalhoursMaximum daily dosemgÆkg )1 Æday )1Licensedfrom ageIbuprofen 5–10 6–8 30 3 monthsDiclofenac 1 8 3 6 monthsKetorolac a 0.5 6 2Naproxen 7.5 12 15Piroxicam a 0.5 24 0.5 N/RKetoprofen a 1 6 4a High <strong>in</strong>cidence of GI complications. Not licensed for acutepa<strong>in</strong>.6.5.2 NSAID toxicity <strong>and</strong> side effectsBecause of their mechanism of action, NSAIDs havethe potential to cause adverse effects at therapeuticplasma levels.l Hypersensitivity reactionsl NSAIDs reduce platelet aggregation <strong>and</strong> prolongbleed<strong>in</strong>g time. Therefore, they are usually contra<strong>in</strong>dicated<strong>in</strong> children with coagulation disorders or <strong>in</strong>those who are receiv<strong>in</strong>g anticoagulant therapy.l NSAIDs can <strong>in</strong>hibit prostagl<strong>and</strong><strong>in</strong>-mediated renalfunction, <strong>and</strong> this effect is greater <strong>in</strong> the presence ofrenal disease <strong>and</strong> dehydration. Ibuprofen has beenshown to reduce the glomerular filtration rate <strong>in</strong>neonates by 20%. NSAIDs should not be adm<strong>in</strong>isteredconcurrently with nephrotoxic agents. Renaltoxicity is low <strong>in</strong> healthy children.l NSAIDs can cause gastric irritation <strong>and</strong> bleed<strong>in</strong>g.They are therefore relatively contra<strong>in</strong>dicated <strong>in</strong> childrenwith a history of peptic ulcer disease. Ibuprofenhas the lowest potential for gastric irritation. The riskof adverse GI effects is low when NSAID use is limitedto 1–3 days <strong>in</strong> the postoperative period; it may be furtherreduced by co-prescription of proton pump <strong>in</strong>hibitors,for example, omeprazole <strong>and</strong> H2 antagonists <strong>in</strong>patients at higher risk. Piroxicam, ketorolac, <strong>and</strong> ketoprofenare known to be especially likely to cause GI sideeffects particularly <strong>in</strong> the elderly. In the UK, piroxicamis no longer licensed for acute <strong>in</strong>dications <strong>and</strong> is subjectto special prescrib<strong>in</strong>g <strong>and</strong> monitor<strong>in</strong>g restrictions.l Ow<strong>in</strong>g to excess leukotriene production, NSAIDshave the potential to exacerbate asthma <strong>in</strong> a predisposedsubset of asthmatics. It is estimated that 2% ofasthmatic children are susceptible to aspir<strong>in</strong>-<strong>in</strong>ducedbronchospasm <strong>and</strong> 5% of this subgroup are likely tobe cross-sensitive to other NSAIDs, that is, 1:1000.The <strong>in</strong>cidence of asthma <strong>in</strong> children is <strong>in</strong>creas<strong>in</strong>g, <strong>and</strong>it is important that children who are not sensitive arenot denied the benefits of NSAIDs. History of previousuneventful NSAID exposure should be established<strong>in</strong> asthmatic children whenever possible.Studies have provided some reassur<strong>in</strong>g data regard<strong>in</strong>gthe safety of short-term use of ibuprofen <strong>and</strong> diclofenac<strong>in</strong> asthmatic children. NSAIDs should be avoided<strong>in</strong> children with severe acute asthma.l NSAIDs should be used with caution <strong>in</strong> childrenwith severe eczema, multiple allergies, <strong>and</strong> <strong>in</strong> thosewith nasal polyps. NSAIDs should be avoided <strong>in</strong> liverfailurel Animal studies us<strong>in</strong>g high doses of Ketorolac demonstrateddelayed bone fusion. This has led to concernthat the use of NSAIDs <strong>in</strong> children may delay boneheal<strong>in</strong>g follow<strong>in</strong>g fracture or surgery. This has notbeen supported by human studies, <strong>and</strong> the analgesicbenefits of short-term NSAID use outweigh the hypotheticalrisk of delayed bone heal<strong>in</strong>g: see section 5.8.l NSAIDs are not currently recommended for analgesia<strong>in</strong> neonates due to concerns that they mayadversely affect cerebral <strong>and</strong> pulmonary blood flowregulation.Of the NSAIDs currently available, ibuprofen has thefewest side effects <strong>and</strong> the greatest evidence to supportits safe use <strong>in</strong> children. In a large community-basedstudy <strong>in</strong> children with fever, the risk of hospitalizationfor GI bleed<strong>in</strong>g, renal failure, <strong>and</strong> anaphylaxis was nogreater for children given ibuprofen than those givenparacetamol (15).6.6 ParacetamolParacetamol is a weak analgesic (16,17). On its own, itcan be used to treat mild pa<strong>in</strong>; <strong>in</strong> comb<strong>in</strong>ation withNSAIDs or a weak opioid such as code<strong>in</strong>e, it can beused to treat moderate pa<strong>in</strong>. Studies have demonstratedan opioid spar<strong>in</strong>g effect when it is adm<strong>in</strong>isteredpostoperatively.6.6.1 Paracetamol preparations, doses, <strong>and</strong>routesParacetamol is available for oral adm<strong>in</strong>istration <strong>in</strong>syrup, tablet, <strong>and</strong> dispersible forms. Follow<strong>in</strong>g oraladm<strong>in</strong>istration, maximum serum concentrations arereached <strong>in</strong> 30–60 m<strong>in</strong>. As the mechanism of action iscentral, there is a further delay before maximum analgesiais achieved. Suppositories are available; however,there is wide variation <strong>in</strong> the bioavailability of paracetamolfollow<strong>in</strong>g rectal adm<strong>in</strong>istration. Studies have demonstratedthe need for higher load<strong>in</strong>g doses (of the76 ª 2012 Blackwell Publish<strong>in</strong>g Ltd, Pediatric Anesthesia, 22 (Suppl. 1), 1–79
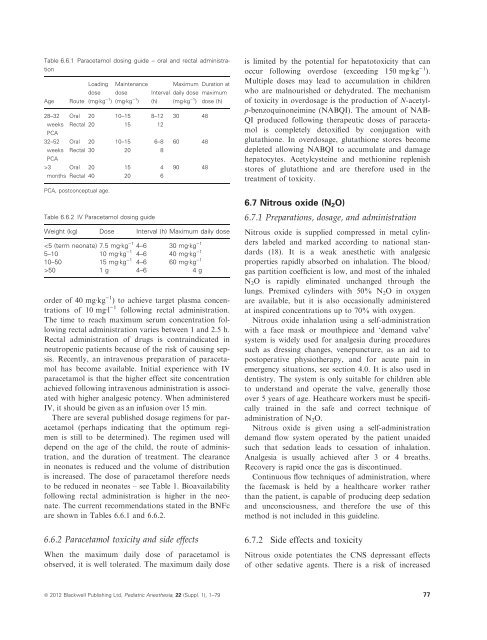
Table 6.6.1 Paracetamol dos<strong>in</strong>g guide – oral <strong>and</strong> rectal adm<strong>in</strong>istrationAgeRouteLoad<strong>in</strong>gdoseMa<strong>in</strong>tenancedoseMaximum Duration atInterval daily dose maximum(mgÆkg )1 ) (mgÆkg )1 ) (h) (mgÆkg )1 ) dose (h)28–32 Oral 20 10–15 8–12 30 48weeks Rectal 20 15 12PCA32–52 Oral 20 10–15 6–8 60 48weeks Rectal 30 20 8PCA>3 Oral 20 15 4 90 48months Rectal 40 20 6PCA, postconceptual age.Table 6.6.2 IV Paracetamol dos<strong>in</strong>g guideWeight (kg) Dose Interval (h) Maximum daily dose50 1 g 4–6 4 gorder of 40 mgÆkg )1 ) to achieve target plasma concentrationsof 10 mgÆl )1 follow<strong>in</strong>g rectal adm<strong>in</strong>istration.The time to reach maximum serum concentration follow<strong>in</strong>grectal adm<strong>in</strong>istration varies between 1 <strong>and</strong> 2.5 h.Rectal adm<strong>in</strong>istration of drugs is contra<strong>in</strong>dicated <strong>in</strong>neutropenic patients because of the risk of caus<strong>in</strong>g sepsis.Recently, an <strong>in</strong>travenous preparation of paracetamolhas become available. Initial experience with IVparacetamol is that the higher effect site concentrationachieved follow<strong>in</strong>g <strong>in</strong>travenous adm<strong>in</strong>istration is associatedwith higher analgesic potency. When adm<strong>in</strong>isteredIV, it should be given as an <strong>in</strong>fusion over 15 m<strong>in</strong>.There are several published dosage regimens for paracetamol(perhaps <strong>in</strong>dicat<strong>in</strong>g that the optimum regimenis still to be determ<strong>in</strong>ed). The regimen used willdepend on the age of the child, the route of adm<strong>in</strong>istration,<strong>and</strong> the duration of treatment. The clearance<strong>in</strong> neonates is reduced <strong>and</strong> the volume of distributionis <strong>in</strong>creased. The dose of paracetamol therefore needsto be reduced <strong>in</strong> neonates – see Table 1. Bioavailabilityfollow<strong>in</strong>g rectal adm<strong>in</strong>istration is higher <strong>in</strong> the neonate.The current recommendations stated <strong>in</strong> the BNFcare shown <strong>in</strong> Tables 6.6.1 <strong>and</strong> 6.6.2.is limited by the potential for hepatotoxicity that canoccur follow<strong>in</strong>g overdose (exceed<strong>in</strong>g 150 mgÆkg )1 ).Multiple doses may lead to accumulation <strong>in</strong> childrenwho are malnourished or dehydrated. The mechanismof toxicity <strong>in</strong> overdosage is the production of N-acetylp-benzoqu<strong>in</strong>oneim<strong>in</strong>e(NABQI). The amount of NAB-QI produced follow<strong>in</strong>g therapeutic doses of paracetamolis completely detoxified by conjugation withglutathione. In overdosage, glutathione stores becomedepleted allow<strong>in</strong>g NABQI to accumulate <strong>and</strong> damagehepatocytes. Acetylcyste<strong>in</strong>e <strong>and</strong> methion<strong>in</strong>e replenishstores of glutathione <strong>and</strong> are therefore used <strong>in</strong> thetreatment of toxicity.6.7 Nitrous oxide (N 2 O)6.7.1 Preparations, dosage, <strong>and</strong> adm<strong>in</strong>istrationNitrous oxide is supplied compressed <strong>in</strong> metal cyl<strong>in</strong>derslabeled <strong>and</strong> marked accord<strong>in</strong>g to national st<strong>and</strong>ards(18). It is a weak anesthetic with analgesicproperties rapidly absorbed on <strong>in</strong>halation. The blood/gas partition coefficient is low, <strong>and</strong> most of the <strong>in</strong>haledN 2 O is rapidly elim<strong>in</strong>ated unchanged through thelungs. Premixed cyl<strong>in</strong>ders with 50% N 2 O <strong>in</strong> oxygenare available, but it is also occasionally adm<strong>in</strong>isteredat <strong>in</strong>spired concentrations up to 70% with oxygen.Nitrous oxide <strong>in</strong>halation us<strong>in</strong>g a self-adm<strong>in</strong>istrationwith a face mask or mouthpiece <strong>and</strong> ‘dem<strong>and</strong> valve’system is widely used for analgesia dur<strong>in</strong>g proceduressuch as dress<strong>in</strong>g changes, venepuncture, as an aid topostoperative physiotherapy, <strong>and</strong> for acute pa<strong>in</strong> <strong>in</strong>emergency situations, see section 4.0. It is also used <strong>in</strong>dentistry. The system is only suitable for children ableto underst<strong>and</strong> <strong>and</strong> operate the valve, generally thoseover 5 years of age. Heathcare workers must be specificallytra<strong>in</strong>ed <strong>in</strong> the safe <strong>and</strong> correct technique ofadm<strong>in</strong>istration of N 2 O.Nitrous oxide is given us<strong>in</strong>g a self-adm<strong>in</strong>istrationdem<strong>and</strong> flow system operated by the patient unaidedsuch that sedation leads to cessation of <strong>in</strong>halation.Analgesia is usually achieved after 3 or 4 breaths.Recovery is rapid once the gas is discont<strong>in</strong>ued.Cont<strong>in</strong>uous flow techniques of adm<strong>in</strong>istration, wherethe facemask is held by a healthcare worker ratherthan the patient, is capable of produc<strong>in</strong>g deep sedation<strong>and</strong> unconsciousness, <strong>and</strong> therefore the use of thismethod is not <strong>in</strong>cluded <strong>in</strong> this guidel<strong>in</strong>e.6.6.2 Paracetamol toxicity <strong>and</strong> side effectsWhen the maximum daily dose of paracetamol isobserved, it is well tolerated. The maximum daily dose6.7.2 Side effects <strong>and</strong> toxicityNitrous oxide potentiates the CNS depressant effectsof other sedative agents. There is a risk of <strong>in</strong>creasedª 2012 Blackwell Publish<strong>in</strong>g Ltd, Pediatric Anesthesia, 22 (Suppl. 1), 1–79 77
- Page 1 and 2:
PediatricAnesthesiaVolume 22 Supple
- Page 3 and 4:
doi: 10.1111/j.1460-9592.2012.3838.
- Page 5 and 6:
1.6 Contact informationCorresponden
- Page 7 and 8:
RecommendationsChildren’s self-re
- Page 9 and 10:
Topical anesthetic preparations, fo
- Page 11 and 12:
2.7.6 Laparoscopic surgeryGood prac
- Page 13 and 14:
In order to assess pain, effective
- Page 16 and 17:
Postoperative painl NCCPC-PV (Non-C
- Page 18 and 19:
68 Broome ME, Richtsmeier A, Maikle
- Page 20 and 21:
however, reductions in the response
- Page 22 and 23:
increased success rate (i.e., less
- Page 24 and 25:
Newer preparations such as liposoma
- Page 26 and 27:
Good practice pointLubricant contai
- Page 28 and 29: most effective. There are a number
- Page 30 and 31: 12 Bellieni C, Bagnoli F, Perrone S
- Page 32 and 33: venipuncture pain in a pediatric em
- Page 34 and 35: 172 van Twillert B, Bremer M, Faber
- Page 36 and 37: necessary to ensure that the patien
- Page 38 and 39: when compared with LA alone and sal
- Page 40 and 41: Peribulbar block improves early ana
- Page 42 and 43: Analgesia Table 5.5.1 Sub-umbilical
- Page 44 and 45: was more effective with less motor
- Page 46 and 47: with using landmark techniques (205
- Page 48 and 49: Good practice pointWound infiltrati
- Page 50 and 51: Analgesia Table 5.6.4 Urological Su
- Page 52 and 53: (298). Ketorolac did not influence
- Page 54 and 55: well as the epidural technique for
- Page 56 and 57: Good practice pointA multi-modal an
- Page 58 and 59: 14 Grainger J, Saravanappa N. Local
- Page 60 and 61: day-stay unit. Int J Paediatr Dent
- Page 62 and 63: tinuous epidural infusion in childr
- Page 64 and 65: 245 Morton NS, O’Brien K. Analges
- Page 66 and 67: 321 Taenzer AH, Clark C, Taenzer AH
- Page 68 and 69: Section 6.0AnalgesiaContents6.1 Ana
- Page 70 and 71: enhance systemic absorption. Lidoca
- Page 73 and 74: undergoes hepatic biotransformation
- Page 75 and 76: tein binding are reduced and the ha
- Page 77: a low-dose infusion but the child m
- Page 81: steps that health care professional