Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
91<br />
Chapter Three<br />
Though <strong>the</strong> reaction was conducted on a small scale, this experiment clearly shows that a �-<br />
allyl complex is formed (peaks at 3.0, 3.8, 4.1 <strong>and</strong> 4.5 ppm in Figure 3.8) <strong>and</strong> subsequently<br />
reacts to form <strong>the</strong> elimination product (133) (peaks at 5.1, 5.3, 6.3 <strong>and</strong> 6.8 ppm in Figure<br />
3.8). Although <strong>the</strong> cationic Pd(II) species proved difficult to separate from (133), dba <strong>and</strong><br />
any excess PPh3, 2-(buta-1,3-dienyl) isoindoline-1,3-dione (133) was obtained as a yellow<br />
solid in 50 % yield.<br />
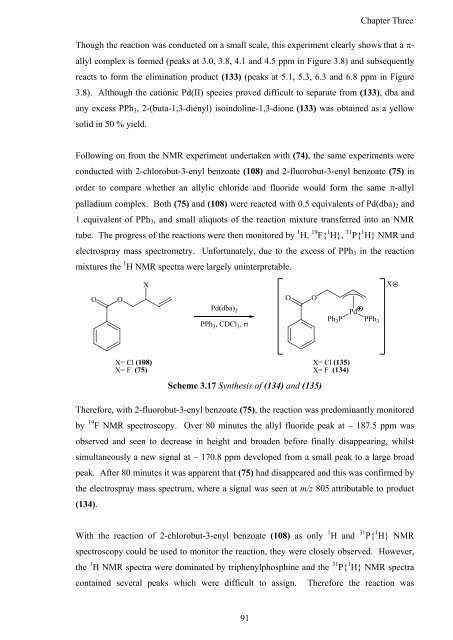
Following on from <strong>the</strong> NMR experiment undertaken with (74), <strong>the</strong> same experiments were<br />
conducted with 2-chlorobut-3-enyl benzoate (108) <strong>and</strong> 2-fluorobut-3-enyl benzoate (75) in<br />
order to compare whe<strong>the</strong>r an allylic chloride <strong>and</strong> fluoride would form <strong>the</strong> same �-allyl<br />
palladium complex. Both (75) <strong>and</strong> (108) were reacted with 0.5 equivalents <strong>of</strong> Pd(dba)2 <strong>and</strong><br />
1 equivalent <strong>of</strong> PPh3, <strong>and</strong> small aliquots <strong>of</strong> <strong>the</strong> reaction mixture transferred into an NMR<br />
tube. The progress <strong>of</strong> <strong>the</strong> reactions were <strong>the</strong>n monitored by 1 H, 19 F{ 1 H}, 31 P{ 1 H} NMR <strong>and</strong><br />
electrospray mass spectrometry. Unfortunately, due to <strong>the</strong> excess <strong>of</strong> PPh3 in <strong>the</strong> reaction<br />
mixtures <strong>the</strong> 1 H NMR spectra were largely uninterpretable.<br />
Scheme 3.17 <strong>Syn<strong>the</strong>sis</strong> <strong>of</strong> (134) <strong>and</strong> (135)<br />
Therefore, with 2-fluorobut-3-enyl benzoate (75), <strong>the</strong> reaction was predominantly monitored<br />
by 19 F NMR spectroscopy. Over 80 minutes <strong>the</strong> allyl fluoride peak at – 187.5 ppm was<br />
observed <strong>and</strong> seen to decrease in height <strong>and</strong> broaden before finally disappearing, whilst<br />
simultaneously a new signal at – 170.8 ppm developed from a small peak to a large broad<br />
peak. After 80 minutes it was apparent that (75) had disappeared <strong>and</strong> this was confirmed by<br />
<strong>the</strong> electrospray mass spectrum, where a signal was seen at m/z 805 attributable to product<br />
(134).<br />
With <strong>the</strong> reaction <strong>of</strong> 2-chlorobut-3-enyl benzoate (108) as only 1 H <strong>and</strong> 31 P{ 1 H} NMR<br />
spectroscopy could be used to monitor <strong>the</strong> reaction, <strong>the</strong>y were closely observed. However,<br />
<strong>the</strong> 1 H NMR spectra were dominated by triphenylphosphine <strong>and</strong> <strong>the</strong> 31 P{ 1 H} NMR spectra<br />
contained several peaks which were difficult to assign. Therefore <strong>the</strong> reaction was