Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
17<br />
Chapter One<br />
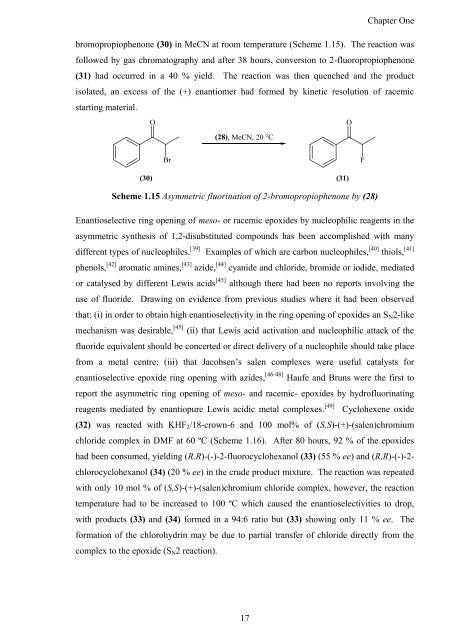
bromopropiophenone (30) in MeCN at room temperature (Scheme 1.15). The reaction was<br />
followed by gas chromatography <strong>and</strong> after 38 hours, conversion to 2-fluoropropiophenone<br />
(31) had occurred in a 40 % yield. The reaction was <strong>the</strong>n quenched <strong>and</strong> <strong>the</strong> product<br />
isolated, an excess <strong>of</strong> <strong>the</strong> (+) enantiomer had formed by kinetic resolution <strong>of</strong> racemic<br />
starting material.<br />
Scheme 1.15 Asymmetric fluorination <strong>of</strong> 2-bromopropiophenone by (28)<br />
Enantioselective ring opening <strong>of</strong> meso- or racemic epoxides by nucleophilic reagents in <strong>the</strong><br />
asymmetric syn<strong>the</strong>sis <strong>of</strong> 1,2-disubstituted compounds has been accomplished with many<br />
different types <strong>of</strong> nucleophiles. [39] Examples <strong>of</strong> which are carbon nucleophiles, [40] thiols, [41]<br />
phenols, [42] aromatic amines, [43] azide, [44] cyanide <strong>and</strong> chloride, bromide or iodide, mediated<br />
or catalysed by different Lewis acids [45] although <strong>the</strong>re had been no reports involving <strong>the</strong><br />
use <strong>of</strong> fluoride. Drawing on evidence from previous studies where it had been observed<br />
that: (i) in order to obtain high enantioselectivity in <strong>the</strong> ring opening <strong>of</strong> epoxides an SN2-like<br />
mechanism was desirable, [45] (ii) that Lewis acid activation <strong>and</strong> nucleophilic attack <strong>of</strong> <strong>the</strong><br />
fluoride equivalent should be concerted or direct delivery <strong>of</strong> a nucleophile should take place<br />
from a metal centre; (iii) that Jacobsen’s salen complexes were useful catalysts for<br />
enantioselective epoxide ring opening with azides, [46-48] Haufe <strong>and</strong> Bruns were <strong>the</strong> first to<br />
report <strong>the</strong> asymmetric ring opening <strong>of</strong> meso- <strong>and</strong> racemic- epoxides by hydr<strong>of</strong>luorinating<br />
reagents mediated by enantiopure Lewis acidic metal complexes. [49] Cyclohexene oxide<br />
(32) was reacted with KHF2/18-crown-6 <strong>and</strong> 100 mol% <strong>of</strong> (S,S)-(+)-(salen)chromium<br />
chloride complex in DMF at 60 ºC (Scheme 1.16). After 80 hours, 92 % <strong>of</strong> <strong>the</strong> epoxides<br />
had been consumed, yielding (R,R)-(-)-2-fluorocyclohexanol (33) (55 % ee) <strong>and</strong> (R,R)-(-)-2-<br />
chlorocyclohexanol (34) (20 % ee) in <strong>the</strong> crude product mixture. The reaction was repeated<br />
with only 10 mol % <strong>of</strong> (S,S)-(+)-(salen)chromium chloride complex, however, <strong>the</strong> reaction<br />
temperature had to be increased to 100 ºC which caused <strong>the</strong> enantioselectivities to drop,<br />
with products (33) <strong>and</strong> (34) formed in a 94:6 ratio but (33) showing only 11 % ee. The<br />
formation <strong>of</strong> <strong>the</strong> chlorohydrin may be due to partial transfer <strong>of</strong> chloride directly from <strong>the</strong><br />
complex to <strong>the</strong> epoxide (SN2 reaction).