Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
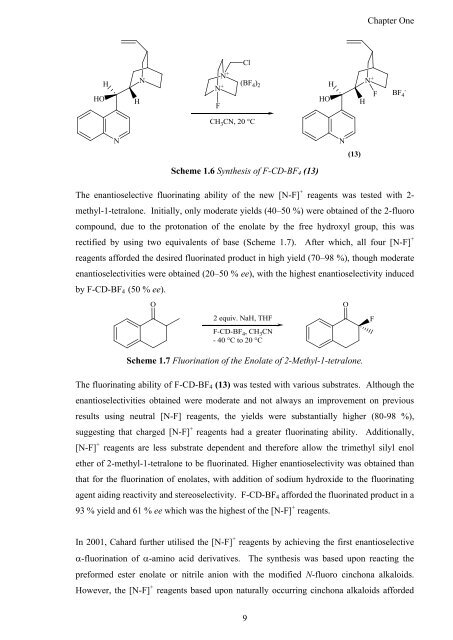
Scheme 1.6 <strong>Syn<strong>the</strong>sis</strong> <strong>of</strong> F-CD-BF4 (13)<br />
9<br />
Chapter One<br />
The enantioselective fluorinating ability <strong>of</strong> <strong>the</strong> new [N-F] + reagents was tested with 2-<br />
methyl-1-tetralone. Initially, only moderate yields (40–50 %) were obtained <strong>of</strong> <strong>the</strong> 2-fluoro<br />
compound, due to <strong>the</strong> protonation <strong>of</strong> <strong>the</strong> enolate by <strong>the</strong> free hydroxyl group, this was<br />
rectified by using two equivalents <strong>of</strong> base (Scheme 1.7). After which, all four [N-F] +<br />
reagents afforded <strong>the</strong> desired fluorinated product in high yield (70–98 %), though moderate<br />
enantioselectivities were obtained (20–50 % ee), with <strong>the</strong> highest enantioselectivity induced<br />
by F-CD-BF4 (50 % ee).<br />
Scheme 1.7 Fluorination <strong>of</strong> <strong>the</strong> Enolate <strong>of</strong> 2-Methyl-1-tetralone.<br />
The fluorinating ability <strong>of</strong> F-CD-BF4 (13) was tested with various substrates. Although <strong>the</strong><br />
enantioselectivities obtained were moderate <strong>and</strong> not always an improvement on previous<br />
results using neutral [N-F] reagents, <strong>the</strong> yields were substantially higher (80-98 %),<br />
suggesting that charged [N-F] + reagents had a greater fluorinating ability. Additionally,<br />
[N-F] + reagents are less substrate dependent <strong>and</strong> <strong>the</strong>refore allow <strong>the</strong> trimethyl silyl enol<br />
e<strong>the</strong>r <strong>of</strong> 2-methyl-1-tetralone to be fluorinated. Higher enantioselectivity was obtained than<br />
that for <strong>the</strong> fluorination <strong>of</strong> enolates, with addition <strong>of</strong> sodium hydroxide to <strong>the</strong> fluorinating<br />
agent aiding reactivity <strong>and</strong> stereoselectivity. F-CD-BF4 afforded <strong>the</strong> fluorinated product in a<br />
93 % yield <strong>and</strong> 61 % ee which was <strong>the</strong> highest <strong>of</strong> <strong>the</strong> [N-F] + reagents.<br />
In 2001, Cahard fur<strong>the</strong>r utilised <strong>the</strong> [N-F] + reagents by achieving <strong>the</strong> first enantioselective<br />
�-fluorination <strong>of</strong> �-amino acid derivatives. The syn<strong>the</strong>sis was based upon reacting <strong>the</strong><br />
preformed ester enolate or nitrile anion with <strong>the</strong> modified N-fluoro cinchona alkaloids.<br />
However, <strong>the</strong> [N-F] + reagents based upon naturally occurring cinchona alkaloids afforded