Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.3 Conclusions<br />
67<br />
Chapter Two<br />
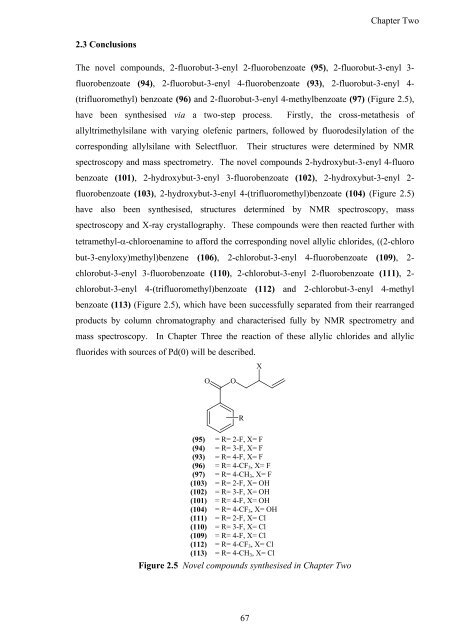
The novel compounds, 2-fluorobut-3-enyl 2-fluorobenzoate (95), 2-fluorobut-3-enyl 3-<br />
fluorobenzoate (94), 2-fluorobut-3-enyl 4-fluorobenzoate (93), 2-fluorobut-3-enyl 4-<br />
(trifluoromethyl) benzoate (96) <strong>and</strong> 2-fluorobut-3-enyl 4-methylbenzoate (97) (Figure 2.5),<br />
have been syn<strong>the</strong>sised via a two-step process. Firstly, <strong>the</strong> cross-meta<strong>the</strong>sis <strong>of</strong><br />
allyltrimethylsilane with varying olefenic partners, followed by fluorodesilylation <strong>of</strong> <strong>the</strong><br />
corresponding allylsilane with Selectfluor. Their structures were determined by NMR<br />
spectroscopy <strong>and</strong> mass spectrometry. The novel compounds 2-hydroxybut-3-enyl 4-fluoro<br />
benzoate (101), 2-hydroxybut-3-enyl 3-fluorobenzoate (102), 2-hydroxybut-3-enyl 2-<br />
fluorobenzoate (103), 2-hydroxybut-3-enyl 4-(trifluoromethyl)benzoate (104) (Figure 2.5)<br />
have also been syn<strong>the</strong>sised, structures determined by NMR spectroscopy, mass<br />
spectroscopy <strong>and</strong> X-ray crystallography. These compounds were <strong>the</strong>n reacted fur<strong>the</strong>r with<br />
tetramethyl-�-chloroenamine to afford <strong>the</strong> corresponding novel allylic chlorides, ((2-chloro<br />
but-3-enyloxy)methyl)benzene (106), 2-chlorobut-3-enyl 4-fluorobenzoate (109), 2-<br />
chlorobut-3-enyl 3-fluorobenzoate (110), 2-chlorobut-3-enyl 2-fluorobenzoate (111), 2-<br />
chlorobut-3-enyl 4-(trifluoromethyl)benzoate (112) <strong>and</strong> 2-chlorobut-3-enyl 4-methyl<br />
benzoate (113) (Figure 2.5), which have been successfully separated from <strong>the</strong>ir rearranged<br />
products by column chromatography <strong>and</strong> characterised fully by NMR spectrometry <strong>and</strong><br />
mass spectroscopy. In Chapter Three <strong>the</strong> reaction <strong>of</strong> <strong>the</strong>se allylic chlorides <strong>and</strong> allylic<br />
fluorides with sources <strong>of</strong> Pd(0) will be described.<br />
(95)<br />
(94)<br />
(93)<br />
(96)<br />
(97)<br />
(103)<br />
(102)<br />
(101)<br />
(104)<br />
(111)<br />
(110)<br />
(109)<br />
(112)<br />
(113)<br />
= R= 2-F, X= F<br />
= R= 3-F, X= F<br />
= R= 4-F, X= F<br />
= R= 4-CF3, X= F<br />
= R= 4-CH3, X= F<br />
= R= 2-F, X= OH<br />
= R= 3-F, X= OH<br />
= R= 4-F, X= OH<br />
= R= 4-CF3, X= OH<br />
= R= 2-F, X= Cl<br />
= R= 3-F, X= Cl<br />
= R= 4-F, X= Cl<br />
= R= 4-CF3, X= Cl<br />
= R= 4-CH3, X= Cl<br />
Figure 2.5 Novel compounds syn<strong>the</strong>sised in Chapter Two