Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
7<br />
Chapter One<br />
The first N-fluorosulfonamide syn<strong>the</strong>sised by Takeuchi <strong>and</strong> Shibata was N-fluoro-3-<br />
cyclohexyl-3-methyl-2,3-dihydrobenzo[1,2-d]-isothiazole-1,1-dioxide (CMIT-F) (8). [21]<br />
This was prepared in five steps, with fluorination in <strong>the</strong> final step proceeding via 15 %<br />
F2/He affording <strong>the</strong> product in a 65 % yield. CMIT-F (8) was reacted with various ketones<br />
to evaluate its enantioselective fluorinating ability, with reactions carried out using 1.1-1.3<br />
equivalents <strong>of</strong> LDA <strong>and</strong> 1.1-1.3 equivalents <strong>of</strong> fluorinating agent in THF solution. The<br />
optimal result was obtained with 2-benzyl-1-tetralone, which yielded <strong>the</strong> fluorinated product<br />
in 79 % <strong>and</strong> 88 % ee. However, all o<strong>the</strong>r enantioselectivities were moderate ranging from<br />
18-74 %.<br />
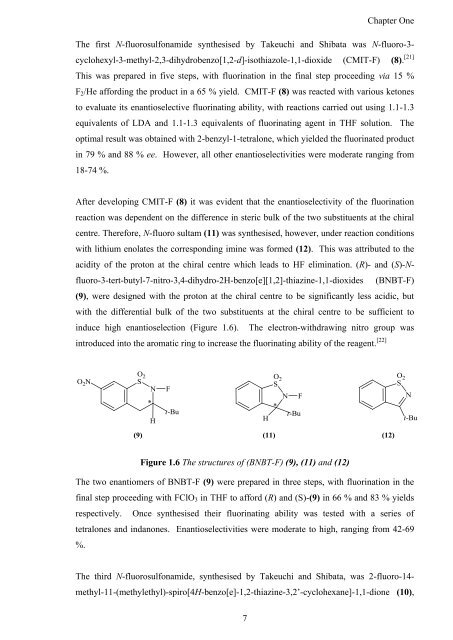
After developing CMIT-F (8) it was evident that <strong>the</strong> enantioselectivity <strong>of</strong> <strong>the</strong> fluorination<br />
reaction was dependent on <strong>the</strong> difference in steric bulk <strong>of</strong> <strong>the</strong> two substituents at <strong>the</strong> chiral<br />
centre. Therefore, N-fluoro sultam (11) was syn<strong>the</strong>sised, however, under reaction conditions<br />
with lithium enolates <strong>the</strong> corresponding imine was formed (12). This was attributed to <strong>the</strong><br />
acidity <strong>of</strong> <strong>the</strong> proton at <strong>the</strong> chiral centre which leads to HF elimination. (R)- <strong>and</strong> (S)-N-<br />
fluoro-3-tert-butyl-7-nitro-3,4-dihydro-2H-benzo[e][1,2]-thiazine-1,1-dioxides (BNBT-F)<br />
(9), were designed with <strong>the</strong> proton at <strong>the</strong> chiral centre to be significantly less acidic, but<br />
with <strong>the</strong> differential bulk <strong>of</strong> <strong>the</strong> two substituents at <strong>the</strong> chiral centre to be sufficient to<br />
induce high enantioselection (Figure 1.6). The electron-withdrawing nitro group was<br />
introduced into <strong>the</strong> aromatic ring to increase <strong>the</strong> fluorinating ability <strong>of</strong> <strong>the</strong> reagent. [22]<br />
Figure 1.6 The structures <strong>of</strong> (BNBT-F) (9), (11) <strong>and</strong> (12)<br />
The two enantiomers <strong>of</strong> BNBT-F (9) were prepared in three steps, with fluorination in <strong>the</strong><br />
final step proceeding with FClO3 in THF to afford (R) <strong>and</strong> (S)-(9) in 66 % <strong>and</strong> 83 % yields<br />
respectively. Once syn<strong>the</strong>sised <strong>the</strong>ir fluorinating ability was tested with a series <strong>of</strong><br />
tetralones <strong>and</strong> indanones. Enantioselectivities were moderate to high, ranging from 42-69<br />
%.<br />
The third N-fluorosulfonamide, syn<strong>the</strong>sised by Takeuchi <strong>and</strong> Shibata, was 2-fluoro-14-<br />
methyl-11-(methylethyl)-spiro[4H-benzo[e]-1,2-thiazine-3,2’-cyclohexane]-1,1-dione (10),