Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.2.3 <strong>Syn<strong>the</strong>sis</strong> <strong>of</strong> <strong>Allyl</strong>ic Chlorides<br />
64<br />
Chapter Two<br />
Once all <strong>the</strong> allylic alcohols had been prepared <strong>the</strong>y could be converted to <strong>the</strong>ir<br />
corresponding chlorides with tetramethyl-�-chloroenamine using <strong>the</strong> method outlined by<br />
Munyemana. [12]<br />
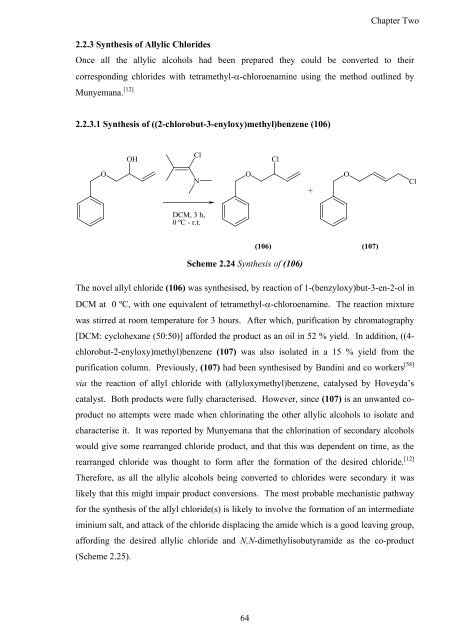
2.2.3.1 <strong>Syn<strong>the</strong>sis</strong> <strong>of</strong> ((2-chlorobut-3-enyloxy)methyl)benzene (106)<br />
Scheme 2.24 <strong>Syn<strong>the</strong>sis</strong> <strong>of</strong> (106)<br />
The novel allyl chloride (106) was syn<strong>the</strong>sised, by reaction <strong>of</strong> 1-(benzyloxy)but-3-en-2-ol in<br />
DCM at 0 ºC, with one equivalent <strong>of</strong> tetramethyl-�-chloroenamine. The reaction mixture<br />
was stirred at room temperature for 3 hours. After which, purification by chromatography<br />
[DCM: cyclohexane (50:50)] afforded <strong>the</strong> product as an oil in 52 % yield. In addition, ((4-<br />
chlorobut-2-enyloxy)methyl)benzene (107) was also isolated in a 15 % yield from <strong>the</strong><br />
purification column. Previously, (107) had been syn<strong>the</strong>sised by B<strong>and</strong>ini <strong>and</strong> co workers [58]<br />
via <strong>the</strong> reaction <strong>of</strong> allyl chloride with (allyloxymethyl)benzene, catalysed by Hoveyda’s<br />
catalyst. Both products were fully characterised. However, since (107) is an unwanted co-<br />
product no attempts were made when chlorinating <strong>the</strong> o<strong>the</strong>r allylic alcohols to isolate <strong>and</strong><br />
characterise it. It was reported by Munyemana that <strong>the</strong> chlorination <strong>of</strong> secondary alcohols<br />
would give some rearranged chloride product, <strong>and</strong> that this was dependent on time, as <strong>the</strong><br />
rearranged chloride was thought to form after <strong>the</strong> formation <strong>of</strong> <strong>the</strong> desired chloride. [12]<br />
Therefore, as all <strong>the</strong> allylic alcohols being converted to chlorides were secondary it was<br />
likely that this might impair product conversions. The most probable mechanistic pathway<br />
for <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> <strong>the</strong> allyl chloride(s) is likely to involve <strong>the</strong> formation <strong>of</strong> an intermediate<br />
iminium salt, <strong>and</strong> attack <strong>of</strong> <strong>the</strong> chloride displacing <strong>the</strong> amide which is a good leaving group,<br />
affording <strong>the</strong> desired allylic chloride <strong>and</strong> N,N-dimethylisobutyramide as <strong>the</strong> co-product<br />
(Scheme 2.25).