Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
3<br />
Chapter One<br />
frequently conducted in ei<strong>the</strong>r high boiling solvents, which aid solubility <strong>of</strong> <strong>the</strong> ionic<br />
fluorides, or in anhydrous solvents, which diminish <strong>the</strong> chances <strong>of</strong> stray hydrogen bonds<br />
forming, which occur due to <strong>the</strong> high hydration energy (123 kcal/mol) <strong>of</strong> fluoride. [13]<br />
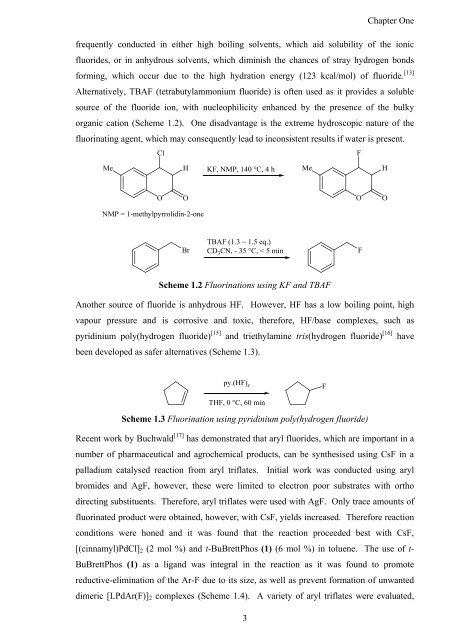
Alternatively, TBAF (tetrabutylammonium fluoride) is <strong>of</strong>ten used as it provides a soluble<br />
source <strong>of</strong> <strong>the</strong> fluoride ion, with nucleophilicity enhanced by <strong>the</strong> presence <strong>of</strong> <strong>the</strong> bulky<br />
organic cation (Scheme 1.2). One disadvantage is <strong>the</strong> extreme hydroscopic nature <strong>of</strong> <strong>the</strong><br />
fluorinating agent, which may consequently lead to inconsistent results if water is present.<br />
Scheme 1.2 Fluorinations using KF <strong>and</strong> TBAF<br />
Ano<strong>the</strong>r source <strong>of</strong> fluoride is anhydrous HF. However, HF has a low boiling point, high<br />
vapour pressure <strong>and</strong> is corrosive <strong>and</strong> toxic, <strong>the</strong>refore, HF/base complexes, such as<br />
pyridinium poly(hydrogen fluoride) [15] <strong>and</strong> triethylamine tris(hydrogen fluoride) [16] have<br />
been developed as safer alternatives (Scheme 1.3).<br />
Scheme 1.3 Fluorination using pyridinium poly(hydrogen fluoride)<br />
Recent work by Buchwald [17] has demonstrated that aryl fluorides, which are important in a<br />
number <strong>of</strong> pharmaceutical <strong>and</strong> agrochemical products, can be syn<strong>the</strong>sised using CsF in a<br />
palladium catalysed reaction from aryl triflates. Initial work was conducted using aryl<br />
bromides <strong>and</strong> AgF, however, <strong>the</strong>se were limited to electron poor substrates with ortho<br />
directing substituents. Therefore, aryl triflates were used with AgF. Only trace amounts <strong>of</strong><br />
fluorinated product were obtained, however, with CsF, yields increased. Therefore reaction<br />
conditions were honed <strong>and</strong> it was found that <strong>the</strong> reaction proceeded best with CsF,<br />
[(cinnamyl)PdCl]2 (2 mol %) <strong>and</strong> t-BuBrettPhos (1) (6 mol %) in toluene. The use <strong>of</strong> t-<br />
BuBrettPhos (1) as a lig<strong>and</strong> was integral in <strong>the</strong> reaction as it was found to promote<br />
reductive-elimination <strong>of</strong> <strong>the</strong> Ar-F due to its size, as well as prevent formation <strong>of</strong> unwanted<br />
dimeric [LPdAr(F)]2 complexes (Scheme 1.4). A variety <strong>of</strong> aryl triflates were evaluated,