Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Synthesis and Comparison of the Reactivity of Allyl Fluorides and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
15<br />
Chapter One<br />
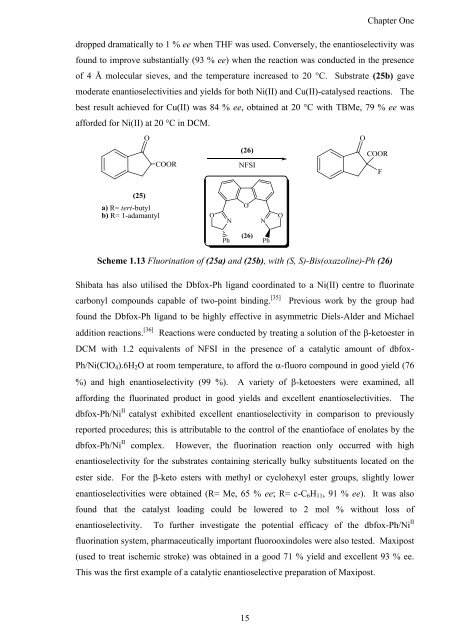
dropped dramatically to 1 % ee when THF was used. Conversely, <strong>the</strong> enantioselectivity was<br />
found to improve substantially (93 % ee) when <strong>the</strong> reaction was conducted in <strong>the</strong> presence<br />
<strong>of</strong> 4 Å molecular sieves, <strong>and</strong> <strong>the</strong> temperature increased to 20 °C. Substrate (25b) gave<br />
moderate enantioselectivities <strong>and</strong> yields for both Ni(II) <strong>and</strong> Cu(II)-catalysed reactions. The<br />
best result achieved for Cu(II) was 84 % ee, obtained at 20 °C with TBMe, 79 % ee was<br />
afforded for Ni(II) at 20 °C in DCM.<br />
Scheme 1.13 Fluorination <strong>of</strong> (25a) <strong>and</strong> (25b), with (S, S)-Bis(oxazoline)-Ph (26)<br />
Shibata has also utilised <strong>the</strong> Dbfox-Ph lig<strong>and</strong> coordinated to a Ni(II) centre to fluorinate<br />
carbonyl compounds capable <strong>of</strong> two-point binding. [35] Previous work by <strong>the</strong> group had<br />
found <strong>the</strong> Dbfox-Ph lig<strong>and</strong> to be highly effective in asymmetric Diels-Alder <strong>and</strong> Michael<br />
addition reactions. [36] Reactions were conducted by treating a solution <strong>of</strong> <strong>the</strong> �-ketoester in<br />
DCM with 1.2 equivalents <strong>of</strong> NFSI in <strong>the</strong> presence <strong>of</strong> a catalytic amount <strong>of</strong> dbfox-<br />
Ph/Ni(ClO4).6H2O at room temperature, to afford <strong>the</strong> �-fluoro compound in good yield (76<br />
%) <strong>and</strong> high enantioselectivity (99 %). A variety <strong>of</strong> �-ketoesters were examined, all<br />
affording <strong>the</strong> fluorinated product in good yields <strong>and</strong> excellent enantioselectivities. The<br />
dbfox-Ph/Ni II catalyst exhibited excellent enantioselectivity in comparison to previously<br />
reported procedures; this is attributable to <strong>the</strong> control <strong>of</strong> <strong>the</strong> enanti<strong>of</strong>ace <strong>of</strong> enolates by <strong>the</strong><br />
dbfox-Ph/Ni II complex. However, <strong>the</strong> fluorination reaction only occurred with high<br />
enantioselectivity for <strong>the</strong> substrates containing sterically bulky substituents located on <strong>the</strong><br />
ester side. For <strong>the</strong> �-keto esters with methyl or cyclohexyl ester groups, slightly lower<br />
enantioselectivities were obtained (R= Me, 65 % ee; R= c-C6H11, 91 % ee). It was also<br />
found that <strong>the</strong> catalyst loading could be lowered to 2 mol % without loss <strong>of</strong><br />
enantioselectivity. To fur<strong>the</strong>r investigate <strong>the</strong> potential efficacy <strong>of</strong> <strong>the</strong> dbfox-Ph/Ni II<br />
fluorination system, pharmaceutically important fluorooxindoles were also tested. Maxipost<br />
(used to treat ischemic stroke) was obtained in a good 71 % yield <strong>and</strong> excellent 93 % ee.<br />
This was <strong>the</strong> first example <strong>of</strong> a catalytic enantioselective preparation <strong>of</strong> Maxipost.