Denver Ascites Shunt PAK 42-2050/42-2055 - CareFusion
Denver Ascites Shunt PAK 42-2050/42-2055 - CareFusion
Denver Ascites Shunt PAK 42-2050/42-2055 - CareFusion
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
en<br />
<strong>Shunt</strong> Description<br />
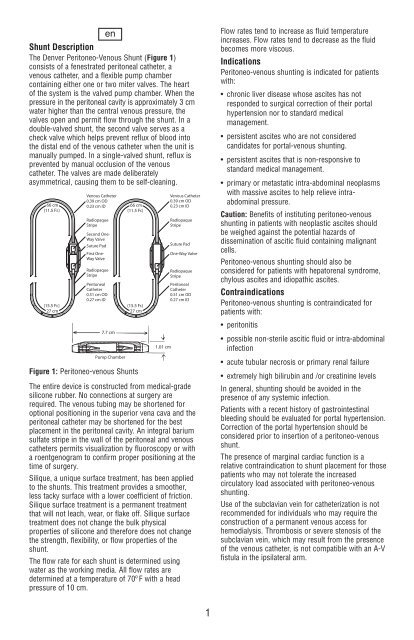
The <strong>Denver</strong> Peritoneo-Venous <strong>Shunt</strong> (Figure 1)<br />
consists of a fenestrated peritoneal catheter, a<br />
venous catheter, and a flexible pump chamber<br />
containing either one or two miter valves. The heart<br />
of the system is the valved pump chamber. When the<br />
pressure in the peritoneal cavity is approximately 3 cm<br />
water higher than the central venous pressure, the<br />
valves open and permit flow through the shunt. In a<br />
double-valved shunt, the second valve serves as a<br />
check valve which helps prevent reflux of blood into<br />
the distal end of the venous catheter when the unit is<br />
manually pumped. In a single-valved shunt, reflux is<br />
prevented by manual occlusion of the venous<br />
catheter. The valves are made deliberately<br />
asymmetrical, causing them to be self-cleaning.<br />
Figure 1: Peritoneo-venous <strong>Shunt</strong>s<br />
The entire device is constructed from medical-grade<br />
silicone rubber. No connections at surgery are<br />
required. The venous tubing may be shortened for<br />
optional positioning in the superior vena cava and the<br />
peritoneal catheter may be shortened for the best<br />
placement in the peritoneal cavity. An integral barium<br />
sulfate stripe in the wall of the peritoneal and venous<br />
catheters permits visualization by fluoroscopy or with<br />
a roentgenogram to confirm proper positioning at the<br />
time of surgery.<br />
Silique, a unique surface treatment, has been applied<br />
to the shunts. This treatment provides a smoother,<br />
less tacky surface with a lower coefficient of friction.<br />
Silique surface treatment is a permanent treatment<br />
that will not leach, wear, or flake off. Silique surface<br />
treatment does not change the bulk physical<br />
properties of silicone and therefore does not change<br />
the strength, flexibility, or flow properties of the<br />
shunt.<br />
The flow rate for each shunt is determined using<br />
water as the working media. All flow rates are<br />
determined at a temperature of 70º F with a head<br />
pressure of 10 cm.<br />
Flow rates tend to increase as fluid temperature<br />
increases. Flow rates tend to decrease as the fluid<br />
becomes more viscous.<br />
Indications<br />
Peritoneo-venous shunting is indicated for patients<br />
with:<br />
• chronic liver disease whose ascites has not<br />
responded to surgical correction of their portal<br />
hypertension nor to standard medical<br />
management.<br />
• persistent ascites who are not considered<br />
candidates for portal-venous shunting.<br />
• persistent ascites that is non-responsive to<br />
standard medical management.<br />
• primary or metastatic intra-abdominal neoplasms<br />
with massive ascites to help relieve intraabdominal<br />
pressure.<br />
Venous Catheter<br />
0.39 cm OD<br />
66 cm<br />
0.23 cm ID<br />
(11.5 Fr.)<br />
Caution: Benefits of instituting peritoneo-venous<br />
Radiopaque<br />
Stripe<br />
shunting in patients with neoplastic ascites should<br />
be weighed against the potential hazards of<br />
Second One-<br />
Way Valve<br />
dissemination of ascitic fluid containing malignant<br />
Suture Pad<br />
cells.<br />
First One-<br />
Way Valve<br />
Peritoneo-venous shunting should also be<br />
Radiopaque<br />
considered for patients with hepatorenal syndrome,<br />
Stripe<br />
chylous ascites and idiopathic ascites. Peritoneal<br />
Catheter<br />
0.51 cm OD<br />
Contraindications<br />
0.27 cm ID<br />
Peritoneo-venous shunting is<br />
(15.5<br />
contraindicated<br />
Fr.)<br />
for<br />
27 cm<br />
patients with:<br />
• peritonitis<br />
7.7 cm<br />
• possible non-sterile ascitic fluid or intra-abdominal<br />
infection<br />
Pump Chamber<br />
• acute tubular necrosis or primary renal failure<br />
• extremely high bilirubin and /or creatinine levels<br />
In general, shunting should be avoided in the<br />
presence of any systemic infection.<br />
Patients with a recent history of gastrointestinal<br />
bleeding should be evaluated for portal hypertension.<br />
Correction of the portal hypertension should be<br />
considered prior to insertion of a peritoneo-venous<br />
shunt.<br />
The presence of marginal cardiac function is a<br />
relative contraindication to shunt placement for those<br />
patients who may not tolerate the increased<br />
circulatory load associated with peritoneo-venous<br />
shunting.<br />
Use of the subclavian vein for catheterization is not<br />
recommended for individuals who may require the<br />
construction of a permanent venous access for<br />
hemodialysis. Thrombosis or severe stenosis of the<br />
subclavian vein, which may result from the presence<br />
of the venous catheter, is not compatible with an A-V<br />
fistula in the ipsilateral arm.<br />
66 cm<br />
(11.5 Fr.)<br />
(15.5 Fr.)<br />
27 cm<br />
1