CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 4<br />
Intensity (Arbitrary units)<br />
O1s (As polished)<br />
Fe 2<br />
O 3<br />
Cr 2<br />
O 3<br />
Cr(OH) 3<br />
535 534 533 532 531 530 529 528 527<br />
Binding energy (eV)<br />
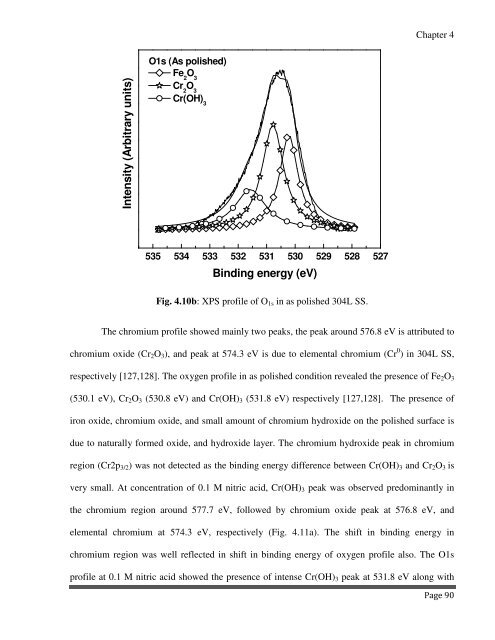
Fig. 4.10b: XPS profile of O 1s in as polished 304L SS.<br />
The chromium profile showed mainly two peaks, the peak around 576.8 eV is attributed to<br />
chromium oxide (Cr 2 O 3 ), and peak at 574.3 eV is due to elemental chromium (Cr 0 ) in 304L SS,<br />
respectively [127,128]. The oxygen profile in as polished condition revealed the presence of Fe 2 O 3<br />
(530.1 eV), Cr 2 O 3 (530.8 eV) and Cr(OH) 3 (531.8 eV) respectively [127,128]. The presence of<br />
iron oxide, chromium oxide, and small amount of chromium hydroxide on the polished surface is<br />
due to naturally formed oxide, and hydroxide layer. The chromium hydroxide peak in chromium<br />
region (Cr2p 3/2 ) was not detected as the binding energy difference between Cr(OH) 3 and Cr 2 O 3 is<br />
very small. At concentration of 0.1 M nitric acid, Cr(OH) 3 peak was observed predominantly in<br />
the chromium region around 577.7 eV, followed by chromium oxide peak at 576.8 eV, and<br />
elemental chromium at 574.3 eV, respectively (Fig. 4.11a). The shift in binding energy in<br />
chromium region was well reflected in shift in binding energy of oxygen profile also. The O1s<br />
profile at 0.1 M nitric acid showed the presence of intense Cr(OH) 3 peak at 531.8 eV along with